Chapter 45 SLE and Infections
Mortality and Infections in Systemic Lupus Erythematosus
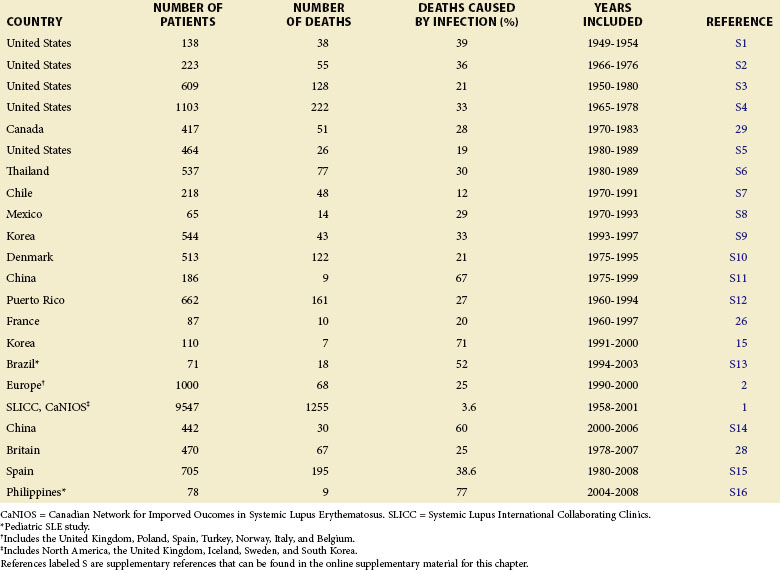
Infection contributes to excess mortality both early and late in the course of SLE and is responsible for approximately 20% to 55% of all deaths in patients with SLE (Table 45-1). A high standardized mortality ratio of 5 for patients with SLE and infection, influenced by gender, age, and disease duration, was reported by Bernatsky and colleagues.1 In a 10-year prospective multinational cohort study of patients with SLE, infections and cardiovascular events were the most frequent cause of death.2 The most frequent infectious complications were pneumonia and sepsis of unknown origin. Pulmonary, abdominal, and genitourinary infections during the first 5 years of follow-up were the leading causes of death in a study by Cervera and associates.2 Parallel studies among patients of Mexican and South African descent also denote infection as the leading cause of mortality.3,4
Multiple factors influence mortality from infection in patients with SLE. African-American, Hispanic-American, North American Indian, Eastern Indian, and black Caribbean patients have increased mortality, compared with Caucasian patients.5–8 Long-term survival of patients of Chinese descent with SLE is comparable to that reported for Caucasians, but it is influenced by the age of onset. In a long-term survival study of an inception cohort of Chinese patients with SLE, survival was significantly worse in patients with late-onset disease (i.e., patients diagnosed after 50 years of age). Survival rates in 5-, 10-, and 15-year studies were 66%, 44%, and 44%, respectively, compared with 92%, 83%, and 80% in the group overall (P < 0.0001).9 Among juvenile patients with SLE, the mortality rate of those hospitalized was associated with sepsis, and infection was an important cause of admission to intensive care units.10 A low serum albumin was a predictor of mortality and was also associated with increased infections.11
Prevalence of Infections in Systemic Lupus Erythematosus
The rate of infection in patients with SLE determines a major disease burden and accounts for a significant portion of total morbidity. In a multicenter population study documenting 16,751 hospitalizations of 8670 patients with SLE over a 3-year period, 2123 were considered potentially avoidable. Pneumonia was the major cause of avoidable hospitalization (40.1%), along with cellulitis (19.3%) and pyelonephritis (5.3%).12 A second large study in Mexico estimates the prevalence of infection at 65% in a series of 473 hospitalized patients with SLE.13 In the intensive care setting, the cause of admission among 104 patients with SLE was infection (61.5%, n = 64), most commonly with pneumonia (67.2%), followed by peritonitis (23.4%), urinary tract infection (17.2%), and central nervous system (CNS) infection (4.7%). Most of these patients (96.9%, n = 62) also had signs of lupus activity.13 Long-term prospective data on 66 patients with SLE, a majority of whom were Caucasian, were collected from a Danish population-based study and recorded 26 patients with infection.14 From these 26 individuals, 20 patients (77%) developed infections before an SLE disease flare, with 1 patient (4%) developing an infection during an SLE flare, and 5 (19%) developing infections after a disease flare, suggesting that infections may trigger increased SLE disease activity in susceptible patients.
A study by Jeong and associates15 in 2009 cites the incidence of infectious disease as 4.4 in 100 patient-years, with a total follow-up duration of 954 years in a case control study of 110 patients. This incidence rate is significantly reduced from the overall infection rate of 59 in 100 and 142 in 100 patient-years cited from other studies during the past decade. Forty-two patients (38%) had at least one episode of an infectious disease, with the type of infection similar to that in the other studies presented in this text but with 32 community-acquired infections versus 10 nosocomial infections. Pathogens were identified in 24 patients, of whom 5 were identified with Streptococcus pneumoniae.15
Identifying Independent Risk Factors for Infection in Systemic Lupus Erythematosus
At this time, no definitive test is available to distinguish SLE disease activity from infection, but several risk factors for infection have been identified (Table 45-2). Yuhara and colleagues16 noted that a model incorporating a decreased serum albumin level increased serum creatinine, and a prednisolone dose equal to or greater than 60 mg per day predicted infection in hospitalized patients with SLE with a sensitivity and specificity of 65% and 91%, respectively. Usually, fever is also a sign of infection and is otherwise rare in patients with SLE receiving prednisone at maintenance doses or greater. Additional risk factors include hypocomplementemia, active nephritis,17 neutropenia18 (although not leukopenia),19 and lymphopenia.20 Other independent predictors of infection at the time of SLE diagnosis are an Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) greater than 12 (p = 0.01), C3 levels less than 90 mg/dL (P = 0.01), and positive anti–double stranded DNA (anti-dsDNA) antibodies (P < 0.01).15 Frequent disease flares (p = 0.04) and follow-up disease duration of 8 years or longer (p = 0.023) were also significant risk factors for increased infections in patients with SLE.15 (See Table 45-2 for additional information regarding a summary of risk factors for infection from a variety of published clinical studies.)15–18,20–32
TABLE 45-2 Identified Risk Factors for Infection in Systemic Lupus Erythematosus Patients
| TYPE OF INFECTION | RISK FACTORS | REFERENCE |
|---|---|---|
| All infections | Active lupus nephritis, disease activity, disease flares, prednisone dose, leukopenia, intravenous steroids and/or immunosuppressive drugs, neutropenia, lymphopenia, complement levels | |
| Major, at time of death | Prednisone dose, cytotoxic drugs, disease activity, disease duration | |
| Fatal opportunistic | Prednisone dose, cytotoxic drugs, complement levels Disease activity | |
| During hospitalization | Disease activity, prednisone dose | |
| Hospitalization for infection | Disease activity, prednisone or prednisolone dose, cytotoxic drugs |
Factors that Influence Infection Susceptibility in Systemic Lupus Erythematosus
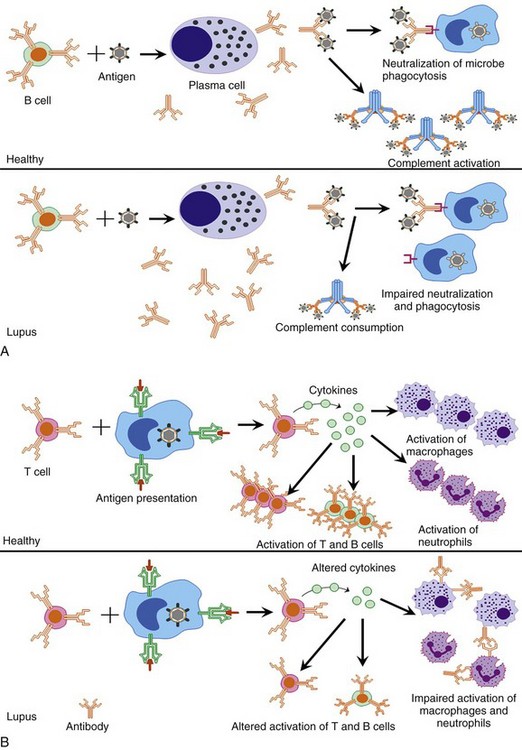
A number of different intrinsic and extrinsic factors are thought to increase patient susceptibility to infection in those with SLE. Patients with SLE have dysregulated immune systems that are often focused on targeting self, rather than protecting against invading pathogens. Defects in macrophages, neutrophils, T cells, natural killer (NK) cells, and B cells may all be critical in the increased risk of infection in SLE patients (Figure 45-1). In addition, select patients with SLE may have defects in immunoglobulin production, complement, and reticuloendothelial pathways that increase the risks for infection. Immunosuppressive actions of a number of standard lupus therapeutic agents also increase the risks for infection. Finally, evolving data suggest that select defects in mannose-binding pathways and fragmented, crystallizable gamma receptor (FcγR) systems may also play roles in the risk for infection in patients with SLE. These issues are briefly discussed in the following text and are reviewed in greater detail in Section two and in the literature.33,34
Systemic Lupus Erythematosus Intrinsic Immune Dysfunction
Macrophages
The monocytes of SLE patients have decreased phagocytic activity and impaired ability to engulf apoptotic cells.35 Superoxide generation is also diminished in these patients after FcγR phagocytosis. Patients with SLE can also have autoantibodies against the FcγRs, as well as genetic polymorphisms within this receptor family, which may decrease the effectiveness of phagocytosis.35 Any or all of these intrinsic immune defects may decrease pathogen clearance and increase the risk of, as well as the ability to respond to, select infections in patients with SLE.
Neutrophils
Neutropenia is a common finding in patients with SLE. Neutrophils in these patients have been shown to have impaired phagocytosis, and this impairment is more pronounced in patients with active disease.36 The neutrophils of these patients also have impaired chemotaxis37 and reduced opsonization.38 These factors combine to decrease effective immune responses against invading pathogens.
T Cells and Natural Killer Cells
Patients with SLE are known to have a variety of T-cell defects. Significant lymphopenia is commonly observed in untreated patients, which can often correlate with increased disease activity. Lymphopenia has also been correlated with increased infectious risk. T cells in these patients have impaired production of a number of cytokines, such as interferon gamma (IFN-γ), interleukin (IL)-1, IL-2, and tumor necrosis factor–alpha (TNF-α), all of which may increase the risk of infection. Extensive discussion of SLE–T cell abnormalities are presented in Chapter 9. Patients with SLE have also been shown to have decreased numbers of NK cells,39 and circulating autoantibodies to NK-cell surface antigens may also contribute to decreased NK-cell activity.40 Impaired T-cell responses in patients with SLE may diminish the ability to respond to viruses and other intracellular pathogens.
B Cells and Immunoglobulin
Autoantibody production, B-cell hyperactivity, and polyclonal B-cell activation are nearly universal in SLE. B-cell abnormalities are described in greater detail in Chapter 8 and Section 3. Some patients with SLE suffer from hypogammaglobulinemia, immunoglobulin (Ig) G subclass deficiencies, IgA deficiencies, or various combinations that could all lead to increased susceptibility to infections.
Reticuloendothelial System Defects
The spleen is the major component of the reticuloendothelial system (RES), and dysfunction of this organ has been described, leading to severe cases of bacterial sepsis.41 Disease activity has also been correlated with defective clearance of IgG-sensitized erythrocytes by the RES.42
Therapeutic Toxicities
Aggressive immunomodulation with glucocorticoids and cytotoxic drugs has dramatically improved the survival rates of patients with SLE. As a consequence, however, severe infections as a result of the chronic immunodeficient state created by these drugs have become major secondary causes of morbidity and mortality. Additionally, patients with SLE are more susceptible to infections than patients with other systemic rheumatic diseases treated with comparable agents. Immunosuppressive drugs used in the treatment of SLE, such as high-dose cyclophosphamide, azathioprine, mycophenolate mofetil, and repeated rituximab,43–45 have been implicated in causing hypogammaglobulinemia, which predisposes patients to a range of infections typically observed in inherited immunodeficiencies.
Glucocorticoids
After 50 years of effective use, glucocorticoids remain a mainstay of lupus therapy. Although their role as powerful immunosuppressants is central to their success as therapeutic agents, the resulting dysregulation of immunity may enhance the susceptibility to the development of infectious disease.46 Glucocorticoids affect cellular function primarily by altering gene regulation via the direct transmission of signals to the nucleus.47 Although the resulting decrease of inflammation is, in fact, the desired therapeutic outcome, both autoinflammatory and pathogen specific immune responses are inhibited by steroid treatment. Glucocorticoids decrease the number of circulating dendritic cells,47 possibly impairing antigen presentation to naive T cells and, subsequently, the responses to new infectious agents. They also inhibit the recruitment of neutrophils and monocyte/macrophages to the inflammatory site and depress monocyte and neutrophil bactericidal activity.
Several studies evaluated the infections in patients with SLE who are receiving glucocorticoid therapy; however, patients with the most active disease usually receive the highest doses of steroids. Prednisolone is a risk factor for the development of CNS infection in patients with SLE if administered at high doses at the onset of infection and high mean doses within the previous year.48 Tam and colleagues49 reported that the cumulative dose, maximum oral dose, and administration by pulse therapy were all independent risk factors for tuberculosis (TB) in patients with SLE who are undergoing steroid therapy. Badsha and colleagues11 showed a high infection rate in the group of patients taking at least 20 mg/day of prednisone for at least 1 month with a history of administration of cyclophosphamide. Low-dose pulse methylprednisolone (1 to 1.5 g over 3 days) was effective in controlling SLE flares and was associated with fewer serious infections than the more traditional high-dose treatment (1 g per day for 3 days). The incidence of infectious complications rises with increased daily doses given for longer than 4 weeks. In one series, a 10 mg per day increase of prednisone increased the risk of serious infection eleven-fold.17 The relative risk ratio for infection was reported to be 1 to 6 in all patients receiving corticosteroid therapy, compared with those not receiving corticosteroids; in addition, alternate-day steroid use considerably reduces the risk of infection.17,50
Other Immunosuppressive Systemic Lupus Erythematosus Therapies
Cyclophosphamide causes neutropenia through both decreased production and increased destruction of neutrophils. Cyclophosphamide use was associated with an increased incidence of herpes zoster infection, and the infection rates in patients receiving the oral and intravenous forms of the drug were found to be comparable.51,52 The rate of infections in patients with SLE and nephritis who were treated with cyclophosphamide plus low-dose steroids is the same as that observed in patients treated with high-dose steroids alone. Pulse cyclophosphamide therapy for lupus nephritis is associated with rates of infections similar to those of daily oral cytotoxic treatment.19
Azathioprine and its metabolite inhibit protein synthesis. Treatment results in lymphopenia and suppressed immunoglobulin synthesis.53 Neutrophil function seems to remain intact with azathioprine therapy; however, neutropenia may result from bone marrow suppression, thus predisposing the patient to infection. This type of neutropenia seems to be dose dependent. Infections complicating cyclosporine therapy are similar to those associated with defective cell-mediated immunity and are thought to arise from impaired transmission of activation signals from the T-cell receptor by calcineurin. Cyclosporine binds to cyclophilin, an endogenous intracellular protein, resulting in a complex that inhibits the activity of calcineurin.54 The incidence of infectious complications appears less frequent with mycophenolate mofetil, an inhibitor of inosine-5′-monophosphate that preferentially inhibits B-cell and T-cell functions, as compared with cyclophosphamide.51 Herpes zoster is still the most common viral infection in patients with SLE who are treated with cyclophosphamides or mycophenolate mofetil.51
Biologic therapies are, by and large, still in the clinical trial phases, and therefore the data on infectious complications from such therapies are limited. A metaanalysis performed by Salliot and associates55 investigated the risk of serious infections of several biologic treatments in SLE. This study did not reveal a significant increase in the risk of serious infection during treatment with rituximab, a peripheral B cell–depleting anti-CD20 monoclonal antibody. Among those patients receiving rituximab who experienced serious infections, bacterial respiratory tract infections were the most common.55 Newer reviews, however, highlight the potential concern for more serious infections with rituximab, including, for example, reactivation of hepatitis B, Pneumocystis infection, or rarely progressive multifocal leukoencephalopathy.56
Belimumab, an anti–B lymphocyte stimulator (anti-BLyS) and B cell–activating factor (BAFF)–directed therapeutic, is the first drug approved by the U.S. Food and Drug Administration (FDA) for SLE in over 50 years. Because belimumab is a new therapeutic drug, few data exist regarding the infection risks in these patients. In a large phase III study of the drug (n = 867), serious infection was reported in 22 patients (8%) receiving 1 mg/kg belimumab, 13 patients (4%) receiving 10 mg/kg belimumab, and 17 patients (6%) receiving a placebo.57 However, real-world use of the medication is needed to determine the clinical rates of infection in anti-BLyS–treated individuals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree