8 Skull Calcifications are a common finding on skull radiographs. With computed tomography, many more calcifications within the skull can be appreciated that escape detection with plain film radiography. Numerous artifacts on the scalp (e.g., dirt, fragments, ointments, and braids) may simulate intracranial calcifications and therefore must be taken into consideration (Fig. 8.1). Physiologic and pathologic intracerebral calcifications occur, although the boundaries between the two can be blurred. Locations of characteristically physiologic calcifications are summarized in Table 8.1 and Fig. 8.2. Table 8.1 Physiologic Intracranial Calcifications
Calcifications
Physiologic Intracranial Calcifications
Pineal |
Habenula |
Choroid plexus |
Dura (falx, tentorium) |
Ligaments (petroclinoid and interclinoid) |
Pituitary |
Internal carotid artery (cavernous portion) |
Basal ganglia and dentate nucleus |
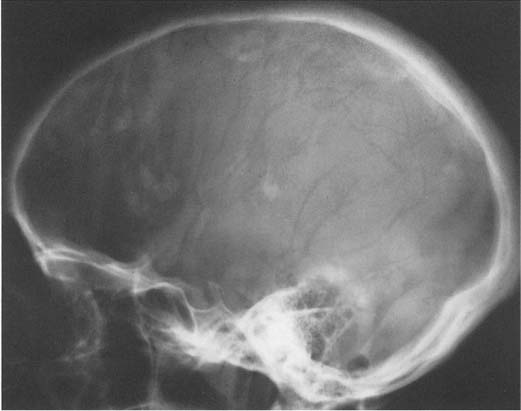
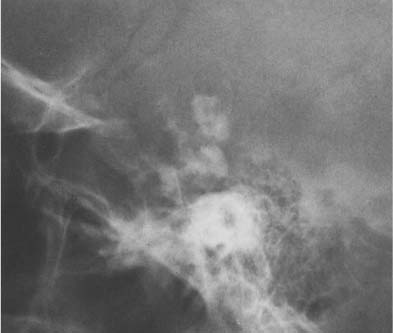
A calcified pineal is found in 5% of children under the age of 10 and in almost two-thirds of the adult population (Fig. 8.3). With CT scanning, a considerably higher rate of pineal calcification is found. It appears amorphous or ring-like, and varies considerably in size but measures usually less than 1 cm. A pineal calcification exceeding 1 cm in diameter suggests neoplasm, either a pinealoma or even more commonly a teratoma. A calcified aneurysm of the vein of Galen may occasionally also simulate an abnormal pineal calcification.
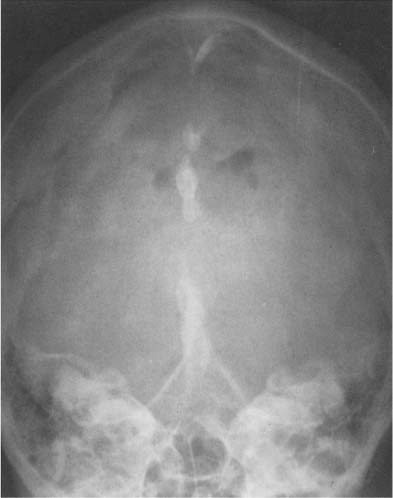
The pineal lies midline in the anteroposterior projection. A displacement of a pineal more than 3 mm to one side of the midline suggests an intracranial mass lesion displacing the pineal away from the midline. On the lateral radiograph, the pineal projects approximately 3 cm above the highest posterior elevation of the pyramids. Numerous methods have been described to assess pineal displacement in this projection, but since their usefulness is rather limited, they will not be discussed in this context.
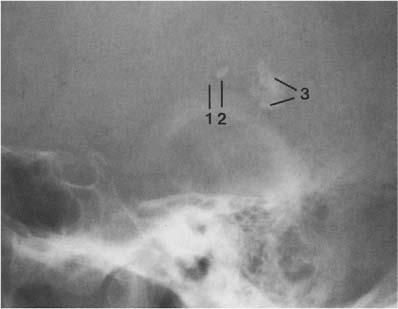
The habenula is located a few millimeters anterior to the pineal and calcifies in almost one-third of patients (Fig. 8.3). Habenular calcification characteristically assumes the shape of a “C” open posteriorly. Habenular displacement by intracranial lesions occurs in the same way as pineal displacement.
Although the choroid plexus can calcify in all ventricles, it most commonly occurs in the atrial portions of the lateral ventricles (junction of the body of the lateral ventricles with the posterior and temporal horns), projecting on the lateral view approximately 2 to 3 cm behind and slightly below the pineal (Fig. 8.3). In the anteroposterior projection, plexus calcifications project approximately 3 cm from the midline and are usually symmetrical, although some disparity in size between the two sides occurs occasionally. The amount of calcification can vary greatly and is of no clinical significance. The calcifications have a characteristically fine to coarse granular appearance, occupying a circular area of 1 cm or more in diameter. Extensive plexus calcifications can be found in neurofibromatosis.
Fig. 8.1 Artifacts. Corn rows (tight African-style braiding of the hair) simulate scattered intracranial calcifications
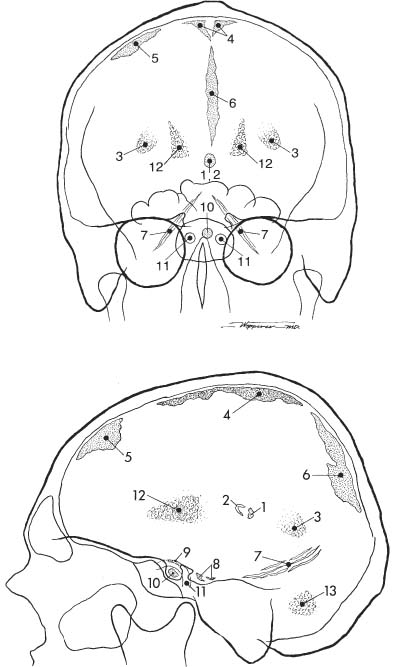
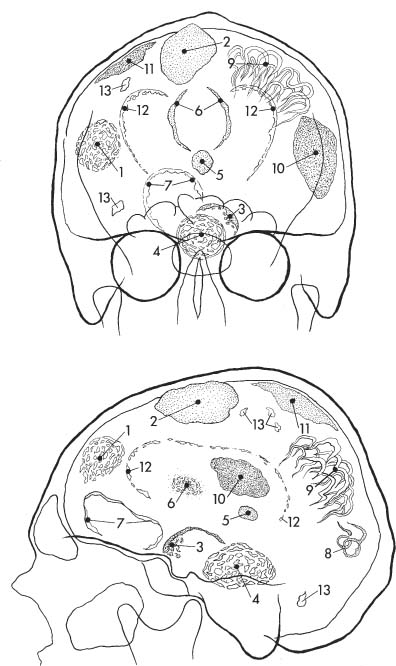
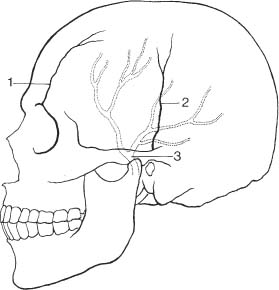
Fig. 8.2a, b Physiologic intracranial calcifications in a anteroposterior and b lateral projection. 1 pineal; 2 habenula; 3 choroid plexus; 4 falx around sagittal sinus; 5 dura; 6 falx (free edge); 7 tentorium; 8 petroclinoid ligament; 9 interclinoid ligament; 10 pituitary; 11 internal carotid artery (cavernous portion); 12 basal ganglia; 13 dentate nuclei.
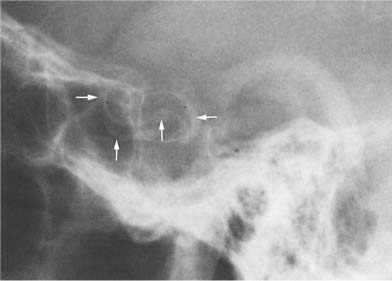
Fig. 8.3 Physiologic intracranial calcifications. From anterior to posterior: 1 C-shaped habenula, 2 pineal gland, and 3 the two superimposed choroid plexuses are seen projecting just above the ear.
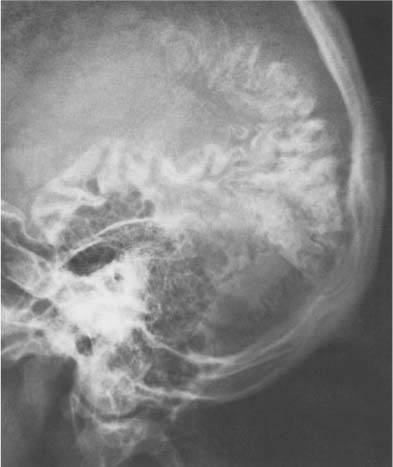
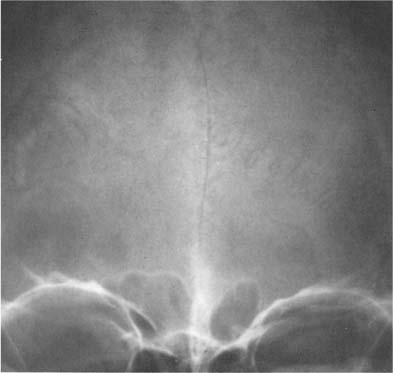
Calcification of dura, falx, and/or tentorium occurs in approximately 10% of cases, and each has quite a characteristic appearance (Fig. 8.4). Dural calcification around the sagittal sinus has a V-shaped appearance at the vertex in the anteroposterior projection. Calcifications in this area may occasionally be caused by calcified Pacchionian (arachnoid) granulations (diverticula-like outpouchings of the arachnoid space penetrating the dura mater and projecting into the lumen of the main sinuses and adjacent venous lakes). Falx calcifications are normally situated anteriorly, and are evident as linear streaks or lamellae in one or both leaves of the falx. Calcifications in the free edge of the tentorium have an inverted V-shape on the anteroposterior projection. The amount of calcification in the dura, falx, and tentorium usually has no clinical significance, particularly when the calcification is more or less diffuse. Falx and dura calcifications have been found in two thirds of patients with basal cell nevus syndrome (Gorlin), and extensive dura calcifications have been reported in pseudoxanthoma elasticum.
Calcifications of the petroclinoid and interclinoid (diaphragma sella) ligaments are common in the elderly. The former lies between the tip of the dorsum sella and the apex of the petrous bone, whereas the latter may result in interclinoid (sellar) bridging.
Pituitary calcifications are rarely recognizable on skull films, as opposed to histologic examinations. On skull films, they may represent calculi.
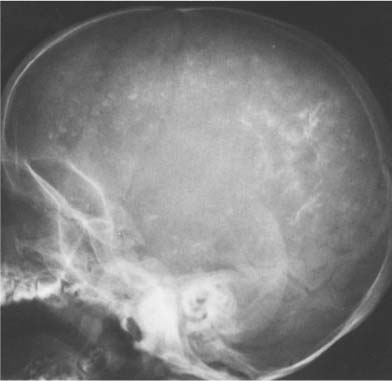
Arteriosclerotic calcifications of the internal carotid artery are commonly seen as it passes through the cavernous sinus. These calcifications can range from a small flake to complete visualization of the carotid syphon (8.5). On the lateral view, these calcifications are superimposed on the sella turcica, whereas ring-like calcifications may be seen on either side of the sella in anteroposterior projection.
Basal ganglia calcifications are found in a number of diseases (see “pathologic calcifications”), but are most often found incidentally in a healthy adult and have no clinical implications. The calcifications range from punctate to conglomerate densities in characteristic locations: on the anteroposterior examinations, the calcifications are symmetrical and parasagittal, whereas on the lateral view, they may assume a gentle curve, roughly paralleling the squamosal suture. However, sclerosis along the squamosal suture, presenting on the lateral view occasionally as a dense band (see Fig. 8.26b), should not be confused with basal ganglia calcifications.
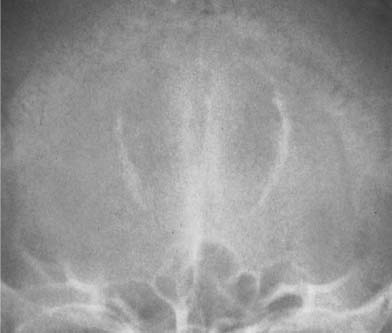
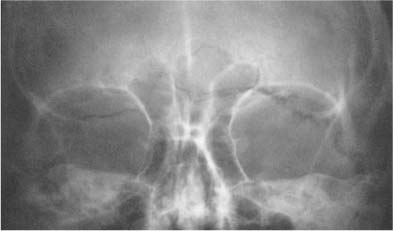
Calcifications in the dentate nucleus of the cerebellum are less common than in the basal ganglia, but are found in the same conditions. On the lateral skull film, these calcifications are often obscured by the mastoid air cells, but are best seen in the occipital (Towne’s) view as symmetrical crescent-shaped densities.
Pathologic Calcifications
Pathologic intracranial calcifications can be subdivided into localized or scattered. Localized calcifications are often suggestive of a specific disease process when both location and shape of the calcification are taken into account. Scattered intracerebral calcifications are virtually limited to a variety of infectious processes, tuberous sclerosis and metastatic carcinomatosis (e. g., from breast carcinoma). Pathologic intracranial calcifications are summarized in Fig. 8.6.
Fig. 8.4 Physiologic Intracranial calcifications. Extensive calcifications of the falx (midline and V-shaped around the sagittal sinus) and tentorium (tent-like above the foramen magnum) are seen. Incidentally, small parasagittal radiolucencies are also noted, representing Pacchionian (arachnoid) granulations.
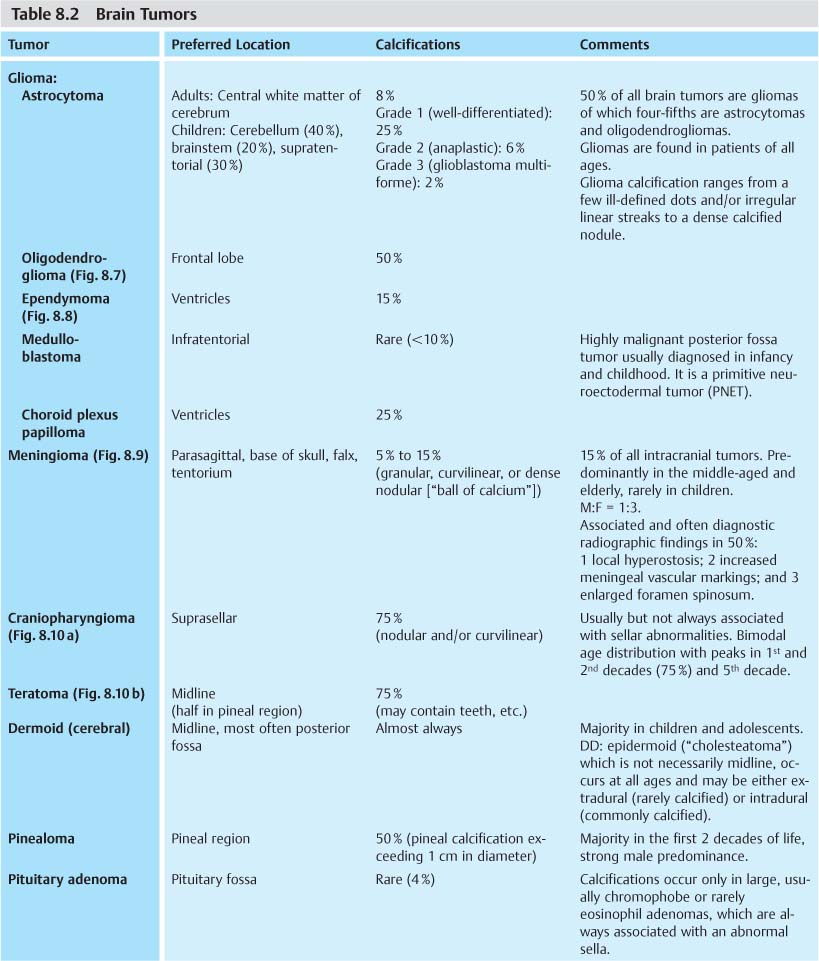
Intracranial tumors represent the largest fraction of localized intracerebral calcifications. Their differential diagnosis is shown in Table 8.2.
Vascular lesions that calcify include (1) aneurysm, (2) arteriovenous malformations, and (3) old hemorrhages (intracerebral, subdural).
Arterial aneurysms occur most commonly in the region of the circle of Willis and calcify in less than 1%. Ring-like or arc-like calcifications are characteristic. Erosion of the adjacent bone may occur. A calcified aneurysm of the vein of Galen is rare, and is usually associated with obstructed hydrocephalus.
Calcifications in arteriovenous malformations are present on skull films in slightly less than 20%. Multiple small peripheral ring shadows combined with scattered flecks or streaks of calcification are almost always pathognomonic. Increased vascular markings in the skull are often an associated radiologic finding. A double-track (“tramline”) calcification in the posterior parietal and/or occipital area is virtually diagnostic of the Sturge-Weber syndrome (meningofacial angiomatosis) (Fig. 8.13). In these cases, an ipsilateral port wine-colored nevus flammeus of the face in the distribution of the trigeminal nerve is almost invariably present. Mental retardation, seizure disorders, and contralateral hemiplegia may also be associated. The ipsilateral hemispheric brain atrophy may be evident radiographically as elevated skull base and compensatory enlargement of the ipsilateral mastoid air cells with increased aeration.
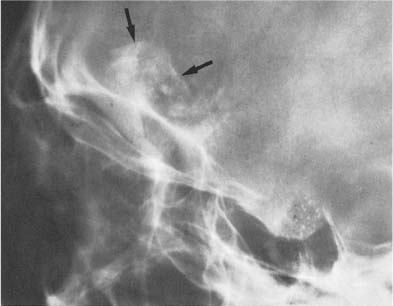
Fig. 8.5 Internal carotid artery calcifications. Both completely calcified carotid syphons (arrows) are superimposed on the sella turcica.
Fig. 8.6a, b Pathologic intracranial calcifications in anteroposterior and lateral projection. 1 Glioma; 2 meningioma; 3 craniopharyngioma; 4 chordoma; 5 pinealoma or teratoma; 6 corpus callosum lipoma; 7 aneurysm; 8 arteriovenous malformation; 9 Sturge-Weber syndrome; 10 old intracerebral hemorrhage (“brain stone”) or granuloma; 11 old subdural or epidural hematoma; 12 cytornegalic inclusion disease or congenital toxoplasmosis; 13 tuberous sclerosis.
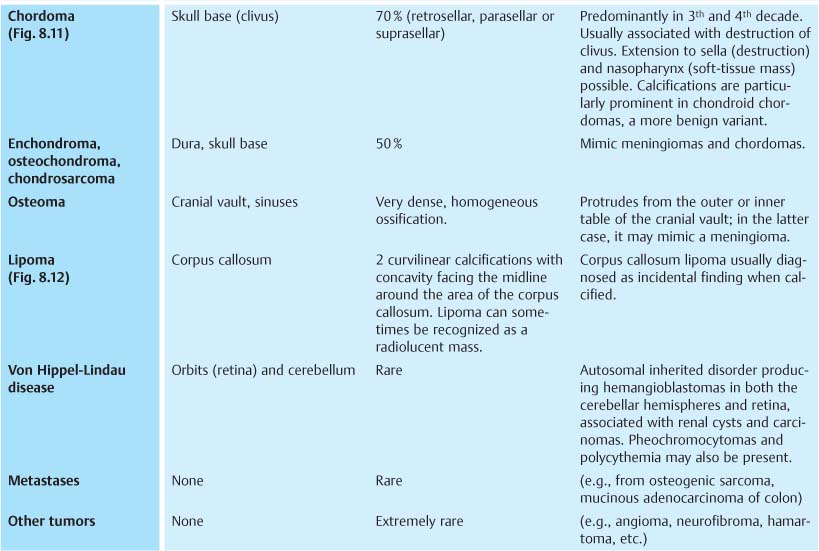
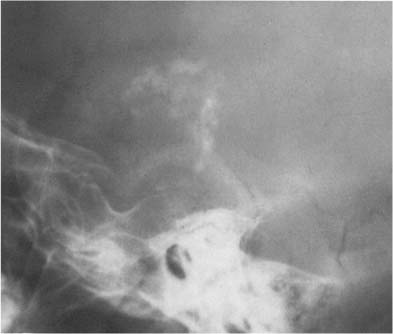
Fig. 8.7 Oligodendroglioma. A tumor calcification is seen in the frontal lobe projecting just above the sphenoid wing (arrows).
Fig. 8.8 Ependymoma. A tumor calcification is seen projecting above the lambdoid suture.
Fig. 8.9 Meningioma. A dense tumor calcification is seen with thickening of the adjacent inner table of the skull.
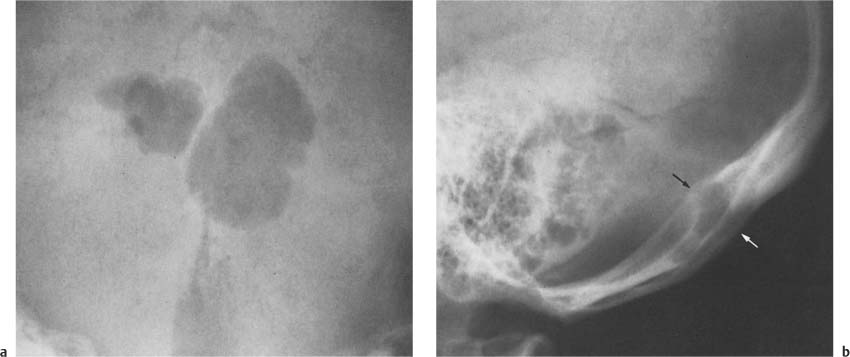
Fig. 8.10a Craniopharyngioma. Nodular tumor calcifications in semicircular configuration are seen above a normal-sized sella. Although sellar abnormalities are commonly associated with this tumor, a normal-sized sella, as in this case, is not unusual in young children. Note the poor definition of the dorsum sellae secondary to increased intracranial pressure.
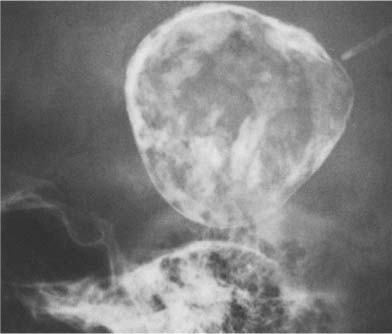
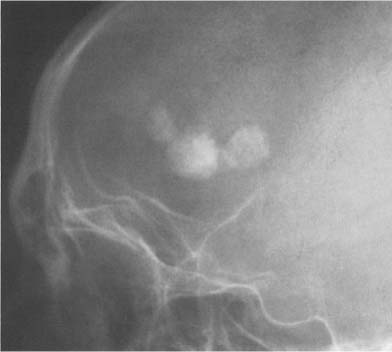
Fig. 8.10b Pineal teratoma. A large calcified mass is seen midline.
Fig. 8.11 Chordoma. Predominantly retrosellar tumor calcifications and destruction of clivus with sellar extension are seen.
Fig. 8.12 Corpus callosum lipoma. Two curvilinear calcifications with the concavity, facing the midline, are diagnostic.
Fig. 8.13 Sturge-Weber syndrome. Extensive double-track (“tramline”) calcifications in the posterior parietal and occipital area extending into the temporal lobes are seen. Ipsilateral large mastoid air cells are also present.
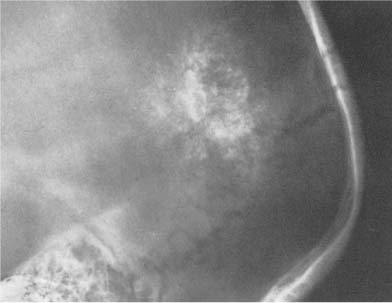
Fig. 8.14 Congenital toxoplasmosis. Scattered calcifications around the enlarged lateral ventricles, characteristically sparing the subtentorial area, are seen.
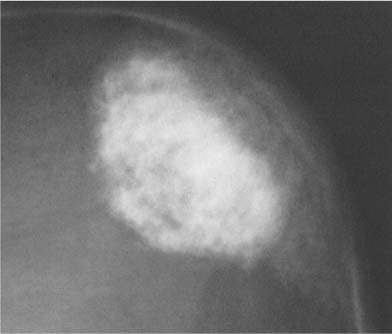
Fig. 8.15 Cryptococcosis. Round calcifications are seen in the frontal lobe area.
Calcifications in intracranial hemorrhages occur. An intracerebral hematoma of either traumatic or spontaneous origin may ultimately result in a dense nodular and amorphous calcification (“brain stone”). Cerebral infarcts may rarely calcify also. Subdural and less frequently epidural hematomas can result on occasion in a thin calcified layer over the hemispheres. The extent of calcification may vary from a small focus to a huge deposit that envelops large portions of one or both hemispheres.
Numerous inflammatory conditions (infections and infestations) may result in intracranial calcifications. When they are located in the brain they are commonly scattered. Congenital cytomegalic inclusion disease is by far the most important diseases in this group, although other viral encephalitides (e.g., polio, herpes, and rubella) have been implicated as a cause of intracerebral calcifications. The incidence of calcification in cytomegalic disease is estimated at approximately 25%. The calcifications are found in the periphery of the often enlarged first and second ventricles.
Calcifications secondary to congenital toxoplasmosis occur in approximately half of the patients, and are virtually indistinguishable from cytomegalic inclusion disease (Fig. 8.14). Other parasitic infestations that can cause scattered intracerebral calcifications are cysticercosis (scattered nodular calcifications 1–3 mm in diameter), trichinosis (punctate calcifications of 1 mm or less) and paragonimiasis (punctate to cystic, often in clusters and measuring up to 3–4 cm in diameter). Rarely, echinococcal disease may cause one or several larger intracranial calcifications.
Tuberculosis is for all practical purposes the only bacterial infection that has to be included in the differential diagnosis of intracranial calcifications. It may present as a single nodule, or less commonly as multiple calcified intracerebral nodules. A healed brain abscess, a syphilitic gumma, or a granuloma caused by a fungal infection (e.g., cryptococcosis, Fig. 8.15) are rare causes of similar localized intracerebral calcifications. Irregular calcifications resulting from tuberculous meningitis are found in the subarachnoid cisterns and project radiographically around and above the sella. Basal arachnoiditis produced by fungal diseases (e.g., coccidioidomycosis) can result in a similar radiographic picture.
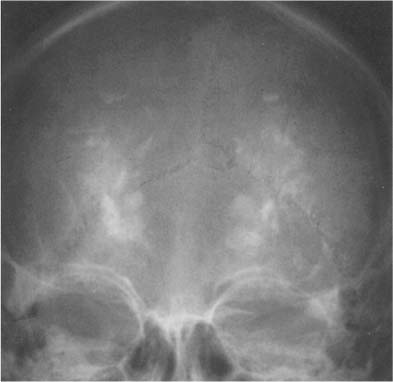
Fig. 8.16 Primary hypoparathyroidism. Extensive calcifications of the basal ganglia are seen. Calcifications of the dentate nuclei were also present, but cannot be recognized in this projection.
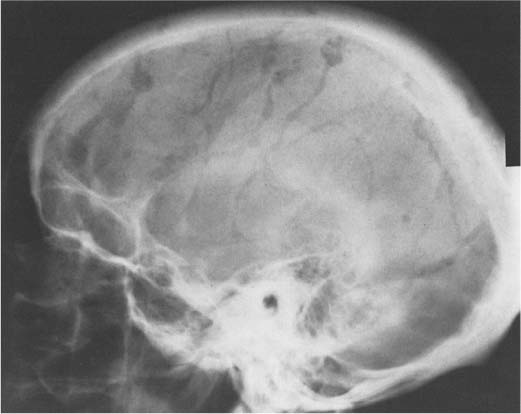
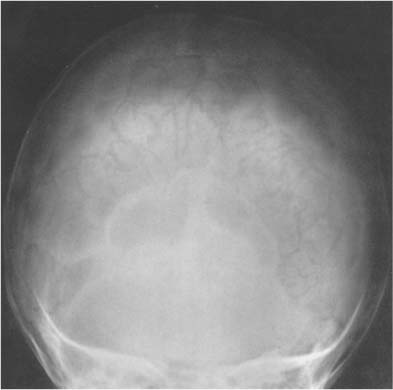
Fig. 8.17 Normal vascular structures. A wide range of radiolucent markings are seen in the skull.
When the basal ganglia and dentate nucleus calcifications are not idiopathic, primary hypoparathyroidism appears to be the most frequent cause (Fig. 8.16), whereas these calcifications are rarely seen following surgical removal of the parathyroids. The calcifications in pseudohypoparathyroidism are radiographically indistinguishable. Calcifications of the basal ganglia and dentate nuclei may also be found in diseases associated with scattered intracerebral calcifications (e.g., tuberous sclerosis, or less commonly toxoplasmosis) and rarely in a few other conditions such as Fahr’s disease (idiopathic familial cerebrovascular ferrocalcinosis), lead and carbon monoxide intoxications, birth anoxia, and certain congenital or acquired neurological disorders.
Vascular Markings, Sutures, and Fracture Lines
Vascular structures are responsible for a wide range of radiolucent markings in the normal skull (Fig. 8.17). With the exception of emissary veins that connect the venous systems inside and outside the skull and may produce bony channels, which are not wider than 2 mm, vascular structures cause indentations only on one table of the skull. Meningeal arteries and veins and dural sinuses produce indentations on the inner table that are fairly constant in position and thus relatively easily recognizable. Pacchionian (arachnoid) granulations, which are arachnoid extensions projecting into the lumen of the main sinuses and adjacent venous lakes, may erode through the inner table into the diploe. They most frequently produce irregular defects in the parasagittal area and the region around the torcula (see Fig. 8.4).
Diploic veins, on the other hand, are extremely variable in size, shape, and number. Besides diploic veins, there are diploic lakes that appear as irregular oval or round radiolucencies, rarely exceeding 2 cm in diameter. Occasionally larger and slightly expansile defects originating from the diploe can be found when a diploic vein forms a larger outpouching (Fig. 8.18). Diploic veins may resemble osteolytic lesions. The demonstration of an irregular and well-demarcated contour, which is characteristic for venous lakes, may be helpful to differentiate them from osteolytic lesions.
Fig. 8.18a, b Venous lakes. Two unusually large well-defined, irregular radiolucencies are seen in the occiput. These large outpouchings of the diploic vein are slightly expansile as seen on the lateral projection (arrows). A single defect would be indistinguishable from an epidermoid originating from the diploe.
The outer table may be indented by the supraorbital artery and the middle branch of the superficial temporal artery. The former is located in the frontal bone above the orbits, whereas the latter runs vertically across the temporal squama and fades out in the inferior part of the parietal bone (Fig. 8.19).
Vascular grooves have to be differentiated from acute fractures, which are usually more radiolucent, since they extend through both the inner and the outer table. Fracture lines also have very sharp and distinct margins (Fig. 8.20). Occasionally a fracture presents as an apparent dense line when the margins overlap in relation to the roentgen beam. This occurs most often with depressed fractures. Sutures may also be confused with acute fractures, when the suture in the outer table with the characteristic serrated appearance is obliterated and only the suture in the inner table remains visible as a relatively straight line. Sutures can, however, be differentiated from fractures by their constant anatomic location, their decreased radiolucency, and their less well-defined margins. Traumatic separation of a suture (diastasis) occurs occasionally in the adult. In children, traumatic suture diastasis has to be differentiated from raised intracranial pressure. In the latter condition, erosion of the dorsum sella, increased convolutional markings, and pineal displacement may also be found. A suture which is normally obliterated can occasionally persist (e.g., the metopic suture in the frontal bone or the mendosal and midsagittal sutures in the occipital bone) and should not be confused with a fracture line (Fig. 8.21).
Wormian bones are small bones occurring within a suture, most commonly within the lambdoidal suture (Fig. 8.22). They have no clinical significance and are found in healthy persons. However, a higher than normal incidence of multiple wormian bones has been found in a variety of congenital disorders such as osteogenesis imperfecta, cretinism (hypothyroidism), cleidocranial dysostosis, progeria, hypophosphatasia, rickets, and many others.
Compared with tubular bones, the osseous healing of skull fractures is slow and often incomplete, with only fibrous tissue formation. Such old fractures may persist as radiolucent lines, which are often difficult to differentiate from vascular markings and sutures.
Fig. 8.19 Arterial grooves on the outer and inner table. They have a constant anatomic location and should not be mistaken for fracture lines. 1 Supraorbital artery (outer table), 2 middle branch of the superficial temporal artery (outer table), and 3 middle meningeal artery (inner table). (Modified from Schunk H, Marayama Y Acta Radiol. 1960; 54: 186).
A localized increase in vascular markings can be a very important finding in the diagnosis of a meningioma when the increased vascular markings are associated with a calcified lesion or a local hyperostosis. It can be found relatively frequently in arteriovenous malformations which are calcified in almost 20% of cases.
Hypervascular primary or secondary tumors of the skull may also be associated with increased vascular markings. They may be observed in Paget’s disease or fibrous dysplasia too, although the radiographic changes in these conditions are usually diagnostic by themselves. Because of a great variation in healthy persons, a generalized increase in the vascular markings is difficult to diagnose, but could indicate collateral circulation in cases with occlusion of major arteries or veins.
Fig. 8.20 Fracture and suture diastasis. Note the sharp and distinct margins of the fracture line projecting into the right orbit and the traumatic separation of the left lambdoid suture, whereas the normal lambdoid suture projecting into the frontal sinus has an indistinct margin and is barely visible.
Fig. 8.21 Metopic suture. This suture is normally obliterated, but may occasionally persist and present as a poorly defined radiolucent line in the middle of the frontal bone, and should not be confused with a fracture.
Fig. 8.22 Wormian bones. Numerous small bones are seen in the lambdoidal suture.
Osteosclerotic Lesions of the Vault of the Skull
For the differential diagnosis, sclerotic changes of the skull vault are best divided into localized and diffuse lesions.
Localized Sclerosis (Single or Multiple Osteoblastic Lesions) of the Skull Vault
As described in more detail in Chapter 2, the differential diagnosis of solitary or multiple localized sclerotic lesions includes benign tumors (e.g., osteoma, osteochondroma), malignant tumors (e.g., osteosarcoma, metastases) (Fig. 8.23), chronic osteomyelitis (Fig. 8.24), ischemic necrosis (especially in bone flaps), radiation osteonecrosis (Fig. 8.25), fibrous dysplasia, neurofibromatosis, Paget’s disease (“cotton wool” appearance), mastocytosis, and tuberous sclerosis (often associated with scattered intracerebral calcifications). Formation of a band-like sclerosis along sutures is relatively common and without any clinical significance. Such a sclerosis along the squamosal suture should not be confused on the lateral view with calcifications in the basal ganglia (Fig. 8.26). Hyperostosis frontalis interna is an idiopathic irregular thickening of the inner table, mainly of the frontal bone (Fig. 8.26). The lesions are characteristically bilateral and symmetrical and spare the midline. They are most commonly found in women over 40 years of age, and progress at a very slow pace over the years. Thickening of the inner tables of other cranial bones or a more generalized thickening of the inner tables occur rarely. The latter condition is called hyperostosis interna generalisata.
An ossified cephalhematoma or subdural hematoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree