CHAPTER 19 Skin substitutes and extracellular matrix scaffolds
1. Differentiate between cellular and acellular products for chronic wound care.
2. Define autologous, allogeneic, xenographic, biosynthetic, and synthetic with regard to skin substitutes and extracellular matrix (ECM) scaffolds.
4. Compare and contrast three types of skin substitutes and the ECM scaffolds in terms of formulation, availability, and role in wound care.
5. Summarize future directions for cell-based therapies.
6. Describe the keys to success when using these products.
7. Describe two best-practice algorithms for the use of skin substitutes in the care of patients with venous leg ulcers and diabetic foot ulcers.
When the patient with a chronic wound has not responded to optimal care, including both systemic support and local wound management, use of advanced products, such as skin substitutes and extracellular matrix (ECM) scaffolds, may be indicated to promote wound healing and closure. The field of tissue engineering has advanced significantly in the last 2 decades to the point where wound care providers have multiple products from which to choose. These products are derived from human, various animal, and synthetic sources, and they may be cellular or acellular. Depending on the material used and how it is processed, wound healing may result in a traditionally expected scar, or it may actually lead to regenerated tissue, in which the original structure and function of the skin and underlying tissue are partially or completely restored (Badylak, 2007; van Winterswijk and Nout, 2007).
Skin substitutes and ECM scaffolds are adjuncts to excellent wound and patient care. The ultimate success in healing a challenging wound with these products depends on meticulous wound bed and patient preparation, including aggressive correction of the underlying wound etiology and comorbidities that affect healing. Checklist 19-1 lists key points to successful use of skin substitutes and ECM scaffolds.
CHECKLIST 19-1 Key Points to Successful Use of Skin Substitutes and Extracellular Matrix Scaffolds
1. Address underlying pathology.
2. Correct systemic factors interfering with wound healing.
3. Identify and treat clinical infection.
4. Debride all necrotic tissue, fibrinous slough, and surrounding callus.
5. Ensure complete contact of product with wound bed.
6. Fenestrate product (if not already packaged that way) to allow wound fluid to escape and prevent accumulation of fluid under product.
7. Keep products without synthetic backings moist.
8. Follow package insert for directions specific to individual products.
Classification of products
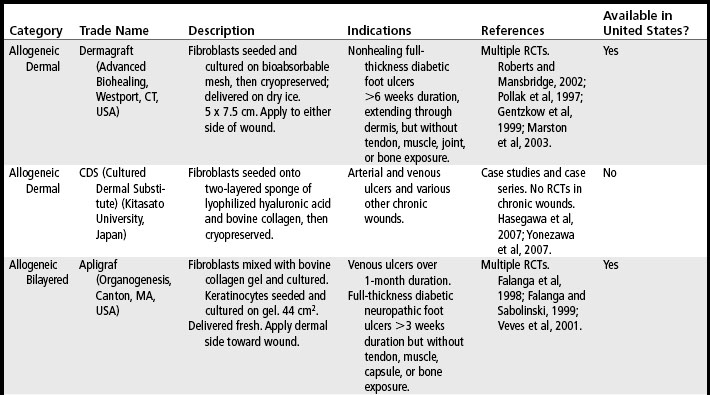
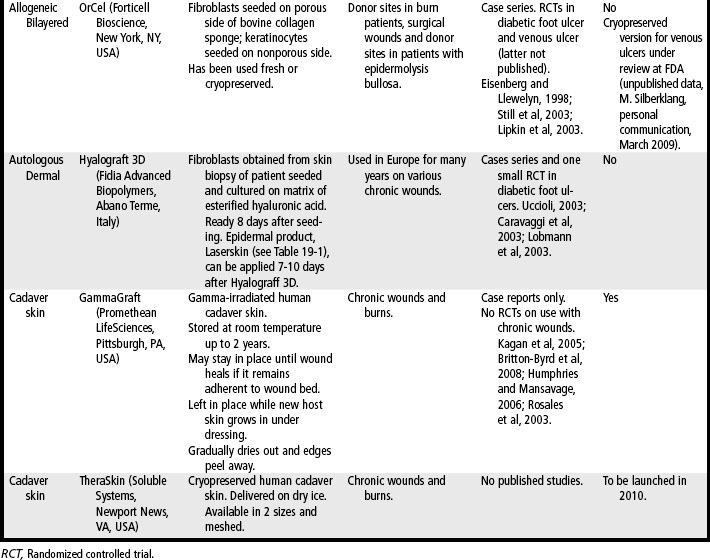
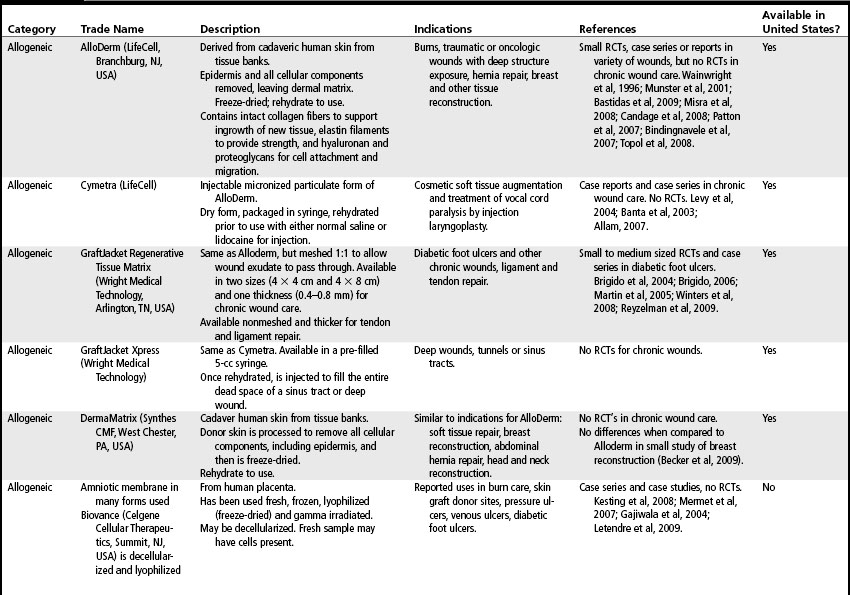
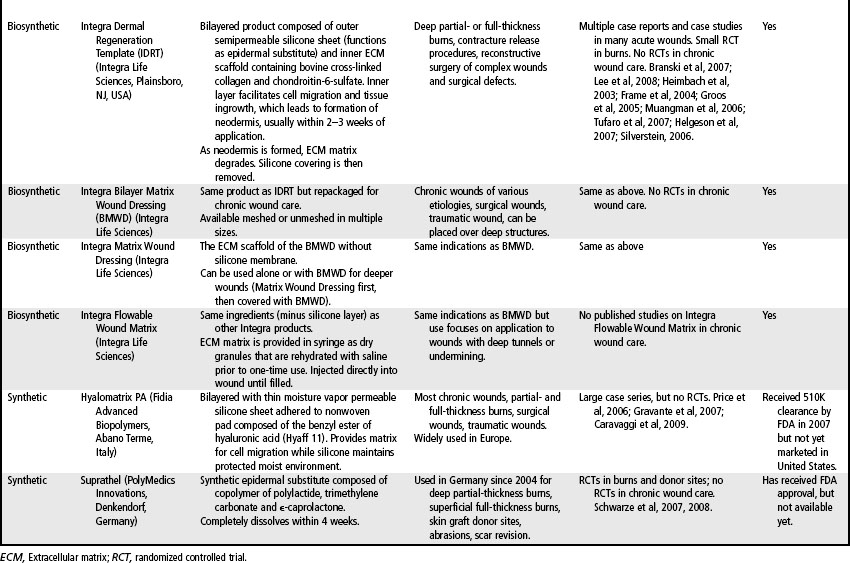
Skin substitutes and ECM products can be classified as cellular (i.e., containing living cells) or acellular (Clark et al, 2007; Shores et al, 2007). Cellular products are frequently referred to as skin substitutes and acellular products as extracellular matrix (ECM) scaffolds. These products may be autologous (derived from the patient’s own body), allogeneic (derived from other humans, also called homografts), xenographic (derived from non-human sources, e.g., porcine, bovine, equine, avian, also called heterografts), biosynthetic (biological and man-made materials), or synthetic (man-made materials) (Ågren and Werthén, 2007; Shores et al, 2007). These products are available as sheets (which may have to be rehydrated), gels, or granules. Some products may retain native growth factors and other polypeptides, such as fibronectin, which promote cell migration and healing in normal wound repair. Processing of skin substitutes and ECM scaffolds will affect their function. For example, products that are engineered to resist degradation will more likely evoke a chronic inflammatory response and, while enhancing wound closure, will result in a scar. In contrast, products that provide controlled degradation while allowing infiltration of and replacement by host tissue have the potential to alter the body’s natural default mechanism to form scar and instead may result in regeneration of tissue layers (Badylak, 2007). Tables 19-1, 19-2, and 19-3 provide examples, descriptions, and indications for known cellular and acellular products, including those that are not available in the United States. In the following sections, products that are available in the U.S. market are presented.
TABLE 19-1 Epidermal Skin Substitutes
| Product | Manufacturer | Description |
|---|---|---|
| Epicel | Genzyme (Cambridge, MA, USA) | Cultured from autologous keratinocytes obtained from skin biopsy of patient. Delivered as 30-cm2 sheets, attached to a petrolatum gauze backing. |
| Laserskin* | Fidia Advanced Polymers (Abano Terme, Italy) | Sheet of esterified hyaluronic acid with laser-created perforations seeded with autologous keratinocytes. |
| Keragraf* | Healthpoint (Fort Worth, Tex., USA) | Cultured from autologous adult stem and precursor cells derived from hair plucked from the patient. |
| Myskin* | Altrika (Sheffield, United Kingdom) | Autologous keratinocytes cultured on silicone sheet with specially formulated surface coating that allows for growth of applied cells. Silicone removed from wound after 1week. New Myskin is applied weekly if needed. |
| CellSpray* | Avita Medical (Cambridge, United Kingdom) | Autologous preconfluent keratinocytes in suspension, sprayed on wound. Requires small biopsy. Suspension ready in 5 days. |
| CellSpray XP* | Avita Medical | Autologous preconfluent keratinocytes in suspension, sprayed on wound. Requires larger biopsy. Suspension ready in 2 days. |
| Bioseed-S* | BioTissue, (Freiberg, Germany) | Autologous keratinocytes suspended in thrombin solution with fibrin sealant. Packaged in syringe. Has shown efficacy in study of 225 patients with venous ulcers (Vanscheidt et al, 2007). |
| Cultured epidermal allografts* | Various; not all commercially available yet | Allogeneic keratinocytes cryopreserved sheets, lyophilized (freeze-dried) sheets, gels, sprays, and suspensions. Studied in leg ulcer care. (Beele et al, 2005; Horch et al, 2005; Navrátilová et al, 2004.) |
* Currently not available in the United States.
Cellular products
Cellular products used in chronic wound care consist of autologous or allogeneic keratinocytes and/or fibroblasts and the ECM proteins and growth factors produced by these cells. Most products consist of confluent cells cultured on various bioabsorbable matrices. They provide cell therapy, defined as “the administration of cells to the body to the benefit of the recipient” (Harris, 2008). Autologous cellular products are expected to “take” or survive. Allogeneic cell-based products, however, rapidly die and slough off (Griffiths et al, 2004; Metcalfe and Ferguson, 2007). The primary purpose of allogeneic products is to deliver healthy living cells to the wound, where they secrete multiple growth factors and other ECM proteins that stimulate the host to proceed with wound repair. The majority of cellular products used in chronic wound care today are produced with allogeneic cells.
Epidermal skin substitutes
One of the first skin substitutes on the market is Epicel (Genzyme, Cambridge, Mass., USA), a cultured epidermal autograft which is indicated for patients with deep dermal to full-thickness burns affecting 30% or greater total body surface area. Epicel is not used in chronic wound care. The main advantage of Epicel in burn care is the ability to cover large wounds with permanent skin that will not be rejected because the graft is autologous (Kamolz, 2008). Enough skin to cover the patient’s entire body can be ready within 4 weeks. Disadvantages to Epicel include the 2-3 week wait for product availability and its fragility. It is only 2-8 cells thick so can be difficult to handle and apply. It also lacks long-term strength which can lead to spontaneous blistering months after grafting (Woodley et al, 1998). Table 19-1 lists other epidermal skin substitutes that are not yet available in the United States.
Dermal skin substitutes
Dermagraft (Advanced Biohealing, Westport, Conn., USA) is a cryopreserved allogeneic dermal substitute with fibroblasts seeded on a bioabsorbable polyglactin mesh (Roberts and Mansbridge, 2002). Once thawed at the bedside in an easy step-by-step process, a 5- × 7.5-cm living dermal substitute can be applied onto clean, debrided wounds that are free of infection.
Dermagraft has demonstrated efficacy in the treatment of patients with diabetic foot ulcers (Gentzkow et al, 1999; Pollak et al, 1997). In a randomized controlled trial of 314 patients with diabetic foot ulcers of over 6 week’s duration, 30% of Dermagraft-treated patients were healed at 12 weeks versus 18.3% in the control group (p = .023) (Marston et al, 2003). Dermagraft is approved by the U.S. Food and Drug Administration (FDA) for the treatment of full-thickness diabetic foot ulcers of greater than 6 weeks duration which extend through the dermis, and are without tendon, muscle, joint capsule or bone exposure. It is also recommended for use in the treatment of nonhealing diabetic foot ulcers by the Wound Healing Society (Steed et al, 2006).
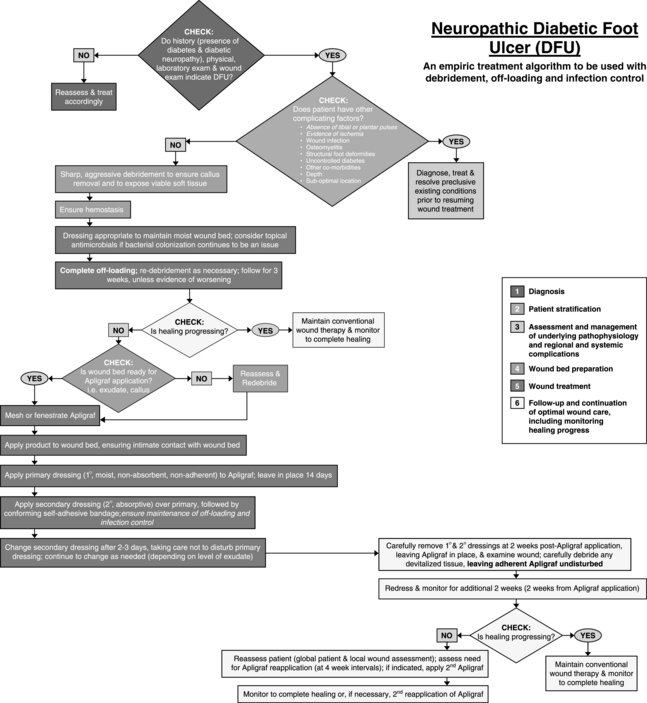
Dermagraft must be used only as an adjunct to standard care, which includes off-weighting the ulcer, debridement, treatment of infection, glucose control, and assurance of adequate blood flow for healing. Optimal healing rates are associated with aggressive offloading, not only with footwear but also with crutches or wheelchairs. Dermagraft is applied with either side toward the wound bed and is covered with a nonadherent contact layer. To ensure good contact of Dermagraft with the wound bed, the wound should be lightly filled with saline-moistened gauze or a soft foam dressing. Although Dermagraft was applied weekly for up to 8 weeks in clinical trials, this frequent application should not be necessary with most patients. The best-practice algorithms published for Apligraf (Figures 19-1 and 19-2) provide excellent guidelines for the use of any skin substitute in the clinical setting (Cavorsi et al, 2006).
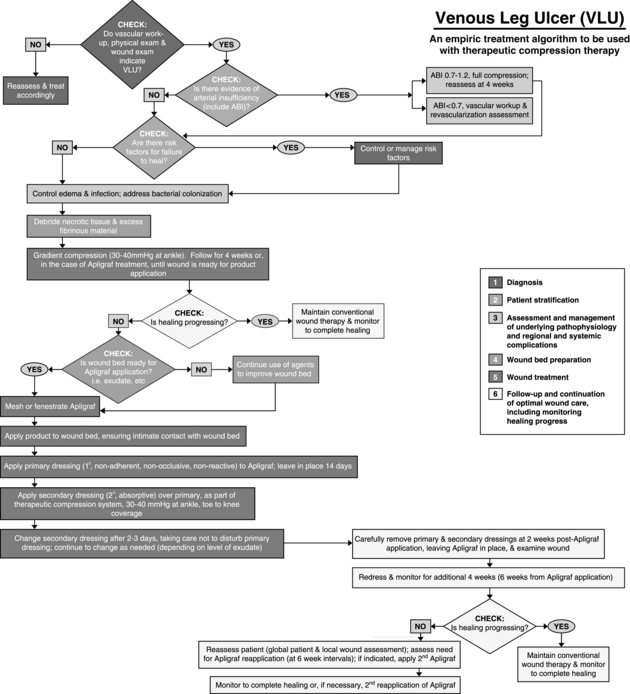
FIGURE 19-1 Best practice algorithm for the use of Apligraf® in the treatment of venous leg ulcers.
(From Cavorsi J et al: Best-practice algorithms for the use of a bilayered living cell therapy (Apligraf®) in the treatment of lower-extremity ulcers, Wound Rep Regen 14:102-109, 2006.)
Bilayered skin substitutes
Apligraf, when used in conjunction with compression therapy, has been demonstrated to achieve closure of hard-to-heal venous ulcers at a significantly higher rate than seen with compression alone (Falanga and Sabolinski, 1999; Falanga et al, 1998). Apligraf is FDA-approved for the treatment of venous ulcers of greater than 1 month duration that have not adequately responded to standard care, including debridement, infection control, assurance of adequate arterial flow, and compression therapy. National guidelines support the use of bi-layered skin substitutes in conjunction with compression bandaging to improve healing rates of venous ulcers (Robson et al, 2006).
Apligraf has also demonstrated efficacy in the treatment of diabetic foot ulcers. In a trial of 208 patients with diabetic neuropathic foot ulcers, Veves et al (2001) found that 56% of patients treated with Apligraf were healed at 12 weeks versus 38% in the control group (p = .0042). Apligraf is FDA-approved for the treatment of diabetic neuropathic foot ulcers of at least 3 weeks duration that extend through the dermis without tendon, muscle, joint capsule, or bone exposure and which have not adequately responded to standard care, including debridement, infection control, glucose control, off-weighting, and assurance of adequate arterial flow.
Like Dermagraft, Apligraf is an adjunct to standard wound care. Apligraf is applied to the wound with the dermal side contacting the wound bed. Many clinicians fenestrate Apligraf with a scalpel prior to application to allow exudate to pass through it onto bandages because pooled exudate under the product may decrease its efficacy. Once applied, it should be anchored with Steri-strips, staples, or skin adhesive. Apligraf should be covered with a nonadherent contact layer and a secondary bandage of choice. Foam dressings work well over venous ulcers because they absorb exudate and apply local pressure under the compression wrap to keep the Apligraf in good contact with the wound bed. Secondary dressings are changed as needed based on the amount of exudate, but the contact layer over the Apligraf should not be disturbed for at least 2 weeks. Any wound cleansing over the ensuing few weeks should be very gentle so as not to disturb any lingering allogeneic cells. Figures 19-1 and 19-2 give algorithms for the use of Apligraf in the clinical setting.
OrCel (Forticell Bioscience, New York, NY, USA) is a bilayered allogeneic product first developed by an Australian physician in the 1980s to treat his son, who suffered from epidermolysis bullosa. Outcomes were improved when OrCel was used in combination with split-thickness autografts in the surgical reconstruction of children with epidermolysis bullosa–related hand contractures and syndactyly (Eisenberg and Llewelyn, 1998). In 2001, the FDA approved the fresh version of OrCel under a humanitarian device exemption (HDE) for the treatment of surgical wounds and donor sites in patients with epidermolysis bullosa undergoing reconstructive hand surgery. Cryopreserved OrCel was later approved with an HDE for the same indication. In a study of split-thickness autograft donor site treatment in burn patients, donor sites treated with Orcel healed an average of 7 days faster versus treatment with Biobrane-L (p < .0006) and were ready for recropping for more autografts an average of 5 days faster (Still et al, 2003). The fresh version of OrCel was FDA approved for the treatment of donor sites in burn patients in 2001. Cryopreserved OrCel has been studied in venous ulcer treatment (unpublished) and FDA approval for this indication is pending. Neither version of Orcel is currently available.
Cadaveric human skin has been used for years as temporary wound coverage in burn care (Britton-Byrd et al, 2008; Kagan et al, 2005). This nonliving product facilitates the short-term coverage of large tissue defects when autografts are not yet available, leading to reduced loss of fluid, electrolytes, and protein, decreased risk of infection, decreased wound desiccation and pain, and improved success of later autografting. One disadvantage of using cadaveric skin is the possibility of disease transmission, despite donor screening regulations by the FDA and quality control standards developed by the American Association of Tissue Banks (AATB) (Humphries and Mansavage, 2006). Another disadvantage is the fact that cadaveric skin is rejected by the host within 2 to 4 weeks. Cadaveric human skin is available fresh (used within 14 days of harvesting), cryopreserved (stored in a freezer for 3–6 months or in liquid nitrogen for up to 10 years), or irradiated (stored at room temperature).
GammaGraft (Promethean LifeSciences, Pittsburgh, Penn., USA) is gamma-irradiated human cadaver skin that provides wound coverage for chronic wounds or burns. It can remain in place until the wound is healed, depending on wound size and depth of tissue damage. GammaGraft is placed on a clean, debrided wound that is free of infection and is covered with a nonadherent dressing for 1 to 2 days. At that time, the graft should be adhered to the wound base. If the graft is not adherent, the wound may be infected, and the graft should be removed. If GammaGraft is adherent, it should be left uncovered; it will dry out and appear much like a scab. It is left in place while new host skin covers the wound underneath. As the wound heals, the edges of the GammaGraft will peel away and can be cut off. Treatment of the underlying problem, such as compression for edema or off-weighting for pressure redistribution, must be continued throughout the treatment period. GammaGraft may be used on any chronic wound. However, although there are case reports of GammaGraft use in chronic wounds (Rosales et al, 2003), no randomized controlled trials have demonstrated the efficacy of this product in wound healing compared to standard care.
Additional products for providing cell therapy in the treatment of chronic wounds are in development (Ehrenreich and Ruszczak, 2006; Hrabchak et al, 2006). Next-generation products may extend beyond the relative simplicity of providing keratinocytes and/or fibroblasts with their secreted growth factors and ECM proteins to a chronic wound. Melanocytes for repigmentation, endothelial cells for neovascularization, stem cells for regeneration of tissue all may be included in future products. Genetically engineered cells may be used that express specific growth factors to stimulate a particular aspect of wound healing (e.g., neovascularization) identified to be deficient in an individual patient. “Smart” skin substitutes may be developed in which genetically engineered cells respond to the wound environment by secreting or restricting secretion of certain cytokines, depending on what the wound requires to progress through repair, or better yet, regeneration (Metcalfe and Ferguson, 2007). Cells can be engineered to secrete increased levels of natural bactericidal chemicals to decrease risk of infection (Schurr et al, 2009). As these products become available, clinicians must critically analyze data to ensure that the use of these products enhances healing while reducing complications compared with standard care.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree