Fig. 18.1
Walch classification of glenoid morphology based on the wear pattern and version. a Type A1—centered humeral head with minor glenoid erosion. b Type A2—centered humeral head with major glenoid erosion. c Type B1—posterior subluxation with no erosion. d Type B2—posterior subluxation and erosion with a biconcave glenoid. e Type C—severe retroversion greater than 25° [56]
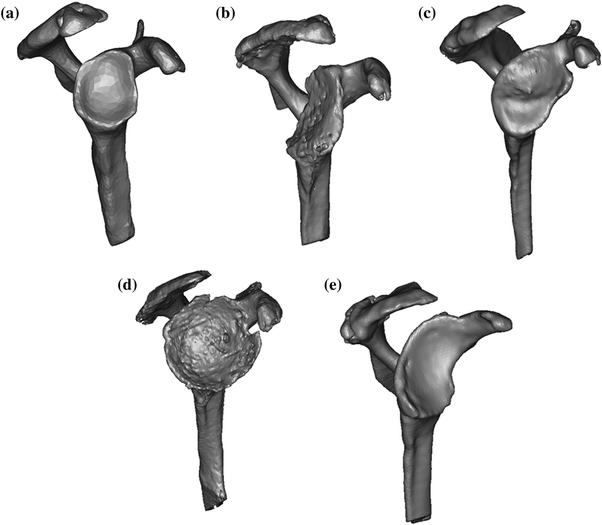
Fig. 18.2
Illustration of the classification of glenoid morphology shows a a normal glenoid, b posterior erosion, c superior erosion, d global erosion, and e anterior erosion [2]
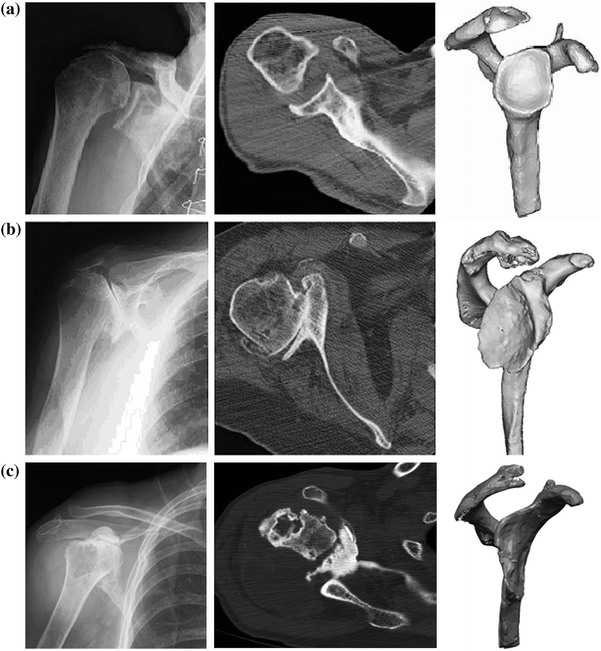
Fig. 18.3
Plain radiograph, axial two-dimensional computed tomography scan, and three-dimensional computed tomography reconstruction model of a shoulder with normal glenoid morphology (a), in a shoulder with abnormal glenoid morphology and posterior bone loss (b), and in a shoulder with abnormal glenoid morphology and anterior bone loss (c) [44]
Techniques to Address Glenoid Bone Loss
The challenge of dealing with glenoid bone loss has been met with many different techniques to either accommodate or correct the deformity and proceed with implantation of an arthroplasty. In the setting of unconstrained total shoulder arthroplasty, a wide range of solutions including preferential reaming, bone grafting techniques, and custom components have been presented. The ideal solution remains elusive as the data continues to be inconsistent. The simplest technique employed involves preferential glenoid reaming. By reaming the “high side,” one can restore a more normal glenoid version. While this is a useful technique in specific circumstances, it is limited by the bone stock available. Studies have suggested that a maximum of 15° of glenoid retroversion can be corrected through reaming alone [24–26]. Excessive reaming increases the risk of peg or keel penetration of the glenoid vault and loss of subchondral bone support, which can ultimately lead to early component failure. Even when this technique is successful in correcting version persistent posterior humeral head subluxation may exist. The results of an unconstrained total shoulder arthroplasty under these circumstances have produced lower functional scores and more pain than average [6].
Augmented glenoid components have also been explored as a solution. Several early designs were reported on and subsequently removed from the market due to poor clinical results [27, 28]. Newer designs using a posterior step-cut prosthesis have shown more encouraging early results than their predecessors [29, 30]; however, nonstandard glenoid components continue to have mixed results [31]. Midterm and long-term results with many of these augmented implant designs are yet unavailable.
Structural bone grafting of significant glenoid defects (Walch type B2 and C) is also an option to restore the bone volume and correct glenoid version. Over time, this has proven to be a complicated and labor-intensive procedure with mixed results. Excellent results were reported by Neer and Morrison [27] in 89 % of patients requiring structural glenoid bone grafts at an average 4.4 years of follow-up. At a mean of 5 years, Steinmann and Cofield [32] reported an 82 % rate of satisfactory results with bone grafting and total shoulder arthroplasty, but 10.7 % of the glenoids were considered radiographically loose. More recently, Sabesan et al. [33] reviewed the clinical results of this treatment in 12 patients at a minimum of 2 years and showed good or excellent results in 83 % of patients. The success of this treatment strategy remains unclear, as further studies have shown less encouraging results. Hill and Norris [34] reported a 47 % unsatisfactory result with a 29 % rate of revision when using bone grafting techniques, and Scalise and Iannotti [35] showed a high rate of graft subsidence. Walch et al. [7] reviewed a large series of patients with biconcave glenoids treated with total shoulder arthroplasty. In this group of patients, total shoulder arthroplasty and posterior bone grafting were associated with worse active elevation, Constant score, mobility, ability to perform activities of daily living, strength, and radiolucent line score. Due to the high complication rate, the authors recommended against this modality of treatment.
Current and historical techniques used in total shoulder arthroplasty have assisted in developing strategies that can be applied to the use of reverse shoulder arthroplasty in the setting of glenoid bone loss. The challenges encountered previously have made it clear that the radiographic and clinical finding of glenoid bone loss is the tip of the iceberg in regard to the complexity of the problem. Surrounding soft tissue contracture has an intricate cause and effect relationship with glenoid bone loss. This may lead to implant failure despite appropriately addressing the bony deformity due to the heavy reliance of an unconstrained arthroplasty on a well-balanced soft tissue envelope. Reverse shoulder arthroplasty may assist in neutralizing these difficult to manage soft tissue imbalances. Mizuno et al. [36] reviewed the results of reverse shoulder arthroplasty in patients treated for primary osteoarthritis with a biconcave glenoid. The functional results for these patients were similar to those in patients treated with total shoulder arthroplasty for primary osteoarthritis as well as those treated with reverse shoulder arthroplasty for rotator cuff deficiency. This may indicate an emerging indication for reverse shoulder arthroplasty as a more reliable solution in general for glenoid bone loss.
While it seems evident that reverse shoulder arthroplasty has the capacity to accommodate more substantial glenoid bone deformity than conventional total shoulder arthroplasty, the greater heterogeneity of pathology in the spectrum of disease treated by reverse shoulder arthroplasty continues to make it a challenge. Strategies employed to deal with these substantial morphologic changes once again include preferential reaming, augmented components, and bone grafting. Over time, we have found that, just as in total shoulder arthroplasty, each of these strategies has its role and its limitations. Preferential reaming plays an important role in providing adequate surface area for the baseplate. This technique is limited by the amount of bone that remains available and may result in loss of adequate bone for baseplate fixation and excessive medialization of the joint line. No data are available regarding the limitations on the amount of correction that can be obtained by reaming alone before fixation is compromised in reverse shoulder arthroplasty. However, as the glenoid surface is medialized the glenoid centerline as described by Matsen et al. [37] becomes effectively shortened. This is the axis used by the central screw or peg in the baseplate. When the depth available for fixation of the central screw is less than 25 mm, recommendations against implantation of a reverse shoulder arthroplasty have been presented due to the increased risk of baseplate failure [38]. Significant medialization of the joint line increases the cosmetic deformity of the shoulder by changing the contour of the deltoid [39, 40]. This has biomechanical implications as well. An excessively medialized humeral component can change the compressive forces of the deltoid into distraction forces, thereby increasing the risk of dislocation [40–42] (Fig. 18.4).
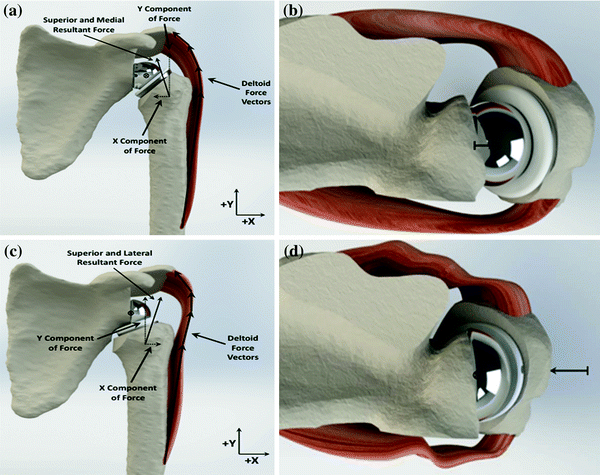

Fig. 18.4
a and b Demonstrate the proper medial–lateral position of the glenosphere in the coronal and axial planes. This produces the proper deltoid tension and vector that creates a compressive force across the component. c and d Illustrate component position in the coronal and axial planes when there is excessive medialization of the glenoid. This creates a lateral force vector for the deltoid which produces a distraction moment across the joint that increases the propensity for dislocation
In order to minimize reaming of the defect, augmented baseplates have been used. While this strategy may be applicable to specific deformities, it is challenging to create a system that adequately addresses each individual deformity one may be presented with. Additionally the central axis of the baseplate still follows the presumed orientation of the standard glenoid centerline. The depth of the bone remaining along this line is less than 15 mm in up to 11 % of the patients with bone loss [43]. This is often inadequate to support stable implant fixation.
Bone grafting of the defect has also been suggested to treat glenoid bone deficiency during the implantation of a reverse shoulder arthroplasty. There is no consensus about when structural graft is necessary for additional baseplate support. Suggestions regarding the threshold for baseplate bone contact before recommending additional bone graft have ranged from 70 to 80 % [22, 36, 44, 45]. Circumstances that require larger bulk graft support present concerns regarding the initial fixation strength to support the component. It is unknown how much host bone is required to supply adequate support. At times, a staged procedure may be necessary to diminish the initial forces across the baseplate–bone interface and allow for integration of the bone graft for load sharing prior to definitive prosthetic implantation. Even when initial fixation seems adequate, the possibility of graft resorption and subsequent implant failure exists. The fate of bulk grafts for the glenoid historically is concerning [46]. In the setting of total shoulder arthroplasty, patients who required glenoid grafting are reported to have poorer clinical outcomes and a relatively high rate of complications due to component failure and graft subsidence [32, 34, 47–51]. Initial data regarding structural grafting of the glenoid in the setting of reverse shoulder arthroplasty has shown more favorable results. These more positive results may indicate that a well-fixed baseplate protects the graft from seeing excessive forces. Kelly et al. [52] showed healing of 11/12 patients treated with glenoid grafting and implantation. Boileau et al. [53] utilized autograft for increased laterization of the joint line and noted 98 % incorporation of the graft and no glenoid component loosening or revisions. Clinical results of grafting glenoid defects in the setting of reverse shoulder arthroplasty have thus far shown low rates of complications and improved function [22, 36, 42, 44, 45, 52, 54] compared to unconstrained total shoulder arthroplasty.
In order to adequately accommodate these complex deformities, one needs a strategy based on maximizing the initial fixation of the baseplate into reliable host bone along with augmented support filling the glenoid defect. The method described initially by Frankle et al. [2] and subsequently detailed by Klein et al. [44] utilizes a technique that changes the axis of orientation of the baseplate to accomplish these goals in a variety of circumstances. Traditionally the central screw or peg of the baseplate is placed along the axis of the glenoid centerline originally described by Matsen et al. [37]. It is defined by a line that originates at the center of the glenoid, perpendicular to the articular surface, and exits on the anterior aspect of the scapular neck (Fig. 18.5). Using a reference axis based on the orientation of the joint surface is problematic when this surface is substantially altered in abnormal glenoids. There is often inadequate bone support along this axis to support fixation of the glenoid component. In order to accommodate the heterogeneous deformities of the glenoid that are encountered, an alternative axis for the baseplate has been defined. The scapular spine centerline is defined by a point originating in the center of the glenoid and is aligned with the axis of the scapular spine and not necessarily perpendicular to the glenoid surface [2, 44] (Fig. 18.5). This axis is anteverted relative to the normal glenoid surface and supplies access to a consistent column of bone and a much greater screw perforation distance. In circumstances where the glenoid is substantially abnormal, the distance to screw perforation of the glenoid vault based on the traditional glenoid centerline can be much shorter. Often times, there is insufficient depth of bone to support initial component fixation. Anteverting the axis along the alternative scapular spine centerline allows consistent access to available host scapular bone for stable initial baseplate fixation. On average, in abnormal glenoids the depth of the glenoid vault is much greater along the axis of the spine centerline compared to the standard glenoid centerline (34.9 vs. 19.8 mm). The amount of anteversion this creates in the component varies dependent on the region of bone loss. In a normal glenoid, the spine centerline is anteverted 9.2° ± 4.9° compared to the standard glenoid centerline. With posterior erosion, the degree of increased anteversion is 20.2° ± 10.4° [2]. This variability has to do with the severity of the erosion causing a relative increased retroversion of the standard glenoid centerline. In other words, the position of the scapular spine centerline stays constant as it relates to the axis of the scapula, while the standard glenoid centerline changes based on the axis perpendicular to the glenoid face. In reviewing the clinical results of the use of the spine centerline to accommodate glenoid bone loss, it has been found that all outcome measures are equal when compared to the patients treated with standard technique in the setting of a morphologically normal glenoid [44]. One could then conclude that the relative anteversion does not have an adverse effect on outcome. Furthermore, the use of this technique effectively neutralizes any potential negative effects of the glenoid deformity on outcome.
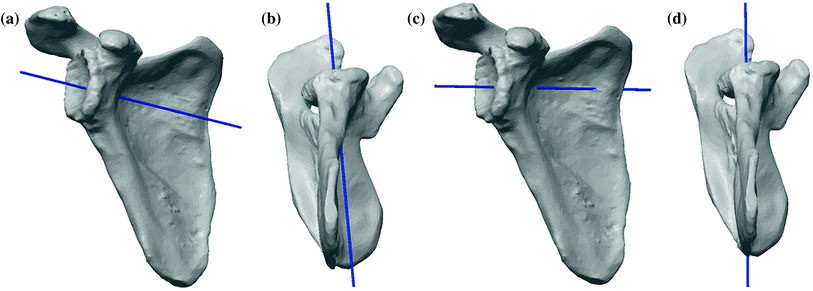

Fig. 18.5
The standard centerline shown in (a) anterior and (b) inferior views, and the alternative centerline along the scapular spine shown in the (c) anterior view and (d) inferior views [2]
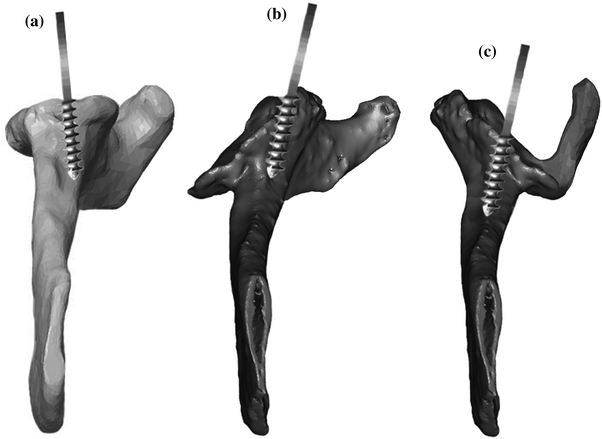
Preferred Method of Treatment
The first step in successful management of a patient with substantial glenoid bone loss treated with reverse shoulder arthroplasty is adequate preoperative planning. Standard plain film radiographs provide valuable information about the condition of the glenoid. When there is a suggestion of increased bone loss, asymmetric wear, medialization to near the coracoid base, evidence of pathologic fracture or a shallow glenoid vault further radiographic characterization of the glenoid is indicated. A CT scan with multiplanar and three-dimensional reconstructions assists the surgeon in preparing properly for what will be encountered during surgery. My preferred technique utilizes a prosthesis with a lateralized center of rotation, anatomic neck angle and version, and a glenoid baseplate with a central screw. Biomechanical advantages of prostheses with this design are detailed earlier in the text (Section “Introduction,” Chap. 13). One advantage of using a device with a central screw is the compression that can be created by the implant against remaining host bone in addition to compressing graft against host bone with the baseplate itself. Locking screws in the baseplate then assist in securing the graft into its compressed position. The surgical approach preferred for these complex reconstructions is a standard deltopectoral approach. This allows access to address all of the pathology present. The humerus is exposed, prepared and eventually implanted in a standard fashion (see Section “Design and Position of the Glenoid,” Chap. 36). The surgical technique for implanting the glenoid component is modified when acquired glenoid defects are encountered [44]. The presence and region of bone loss is determined by a combination of the patient’s preoperative radiographic evaluation and by direct intraoperative inspection. In these cases, the most fundamental alteration in technique is changing the axis of orientation of the central screw to follow the alternative spine centerline. To accomplish this, the glenoid is exposed fully with an aggressive peri-glenoid capsular release. The humerus is retracted posteriorly. Meticulous capsular and scar release obviate the need to disturb either the deltoid or pectoralis major attachments. Orientation of the glenoid and the scapula are assessed by referencing the preoperative radiographs, inspection of the glenoid surface, direct viewing of the coracoid base and by palpation of the scapular neck anteriorly. A central hole using a long 2.5-mm drill is drilled along the alternative spine centerline. It is important to remember that this axis is anteverted anywhere from 10° to 30° from the standard centerline depending on the degree of bone loss present. Often times, it is helpful to palpate the anterior scapular neck all the way to its intersection with the scapular body to assist in the proper orientation of drill. Next, a depth gauge is used to confirm a minimum screw length of 25 mm and the tap is placed (Fig. 18.6). Cannulated convex reamers then prepare the glenoid surface. Due to the anteverted position in which the tap is placed, there will be a preferential amount of anterior glenoid bone removed. In some cases of bone loss, this preferential reaming alone creates a flush surface for baseplate insertion. If less than 80 % of the underside of the glenoid baseplate is in contact with the reamed glenoid because of eccentric bone loss, bulk auto- or allograft is prepared. A reamer that approximates the diameter of the baseplate can be used to estimate the amount of backside contact. The graft is contoured along the backside to fit into the defect. One should ensure that the graft is prepared to bleeding bone to assist ingrowth. Additionally, the sclerotic bone within the glenoid defect can be prepared with a burr and/or 2-mm drill to create a more accepting surface for graft healing. Kirschner wires secure the graft into the defect. The construct is then carefully reamed to conformity with the tap still in place. The tap is removed, and the baseplate is inserted and tightened to compress the graft into the native glenoid surface. Four 5-mm peripheral locking screws are placed in the baseplate to assist in securing the graft and the Kirschner wires are removed. A burr is then used to sculpt the graft and avoid prominence around the periphery of the implant. The choice of glenosphere is affected by the presence of bone graft. Larger-sized hooded glenospheres may be chosen to overhang and protect the bulk graft. Larger glenospheres can increase overall implant–bone contact area diminishing micromotion at the interface and thereby improving baseplate stability [55]. In a study by Klein et al. [44], the larger hooded glenospheres were selected in 46 % of patients. Final glenosphere selection is ultimately determined by the stability, range of motion and soft tissue (including neurovascular structures) tension of the shoulder. When able, however, a sphere that overhangs the graft is chosen (Fig. 18.7). Wound closure is standard and in layers. Subscapularis re-attachment is according to surgeon preference. Immobilization and rehabilitation progresses in a standard fashion and, in the end, bone loss does not affect the postoperative plan or expectations when treated in this fashion.