Fig. 9.1
Schematic of the shoulder simulator. The scapula was potted and rigidly mounted to the simulator such that the glenohumeral joint approximated anatomic orientation. Actuators applied displacement to the deltoid insertion to abduct the arm in the scapular plane, while load cells recorded force. Static loads were applied to the insertions of the rotator cuff muscles to seat the humerus on the glenoid. The elbow was locked in straight or flexed positions with custom external fixation. Arm kinematics were quantified by 3D optical tracking diode arrays on the fixation pins [32]
The three deltoid lines are routed through custom Delrin (DuPont, Wilmington, DE, USA) pulleys rigidly suspended from the simulator frame. A slotted Delrin guide allows the deltoid lines a lateral degree of freedom (<5 mm) along the pulley-bearing surface to prevent binding or dislocation of the dynamically changing lines of action. The anterior deltoid pulley is positioned 5 mm lateral to the anterolateral corner of the coracoid. The middle deltoid pulley is positioned 5 mm lateral to the acromion, midway between the anterolateral and posterolateral corners. The posterior deltoid pulley is positioned 5 mm superior to the scapular spine midway along the insertion of the posterior deltoid. Anatomic landmarks are located by palpation to maintain soft tissue coverage. Rotator cuff lines of action for static loading are applied to the SSC, SS, and IS/TM, where the lines of action are routed over pulleys attached to the embedding block. Static weights are applied to the rotator cuff lines to reduce the joint, allowing the deltoid to act as the prime mover. As noted previously, the simulator currently includes powered rotator cuff lines of action. Each deltoid line of action includes a 100 or 250 lb. load cell to monitor the force applied to achieve the arm motion. The custom control code is written using LabVIEW software (National Instruments Corp, Austin, TX, USA).
In teaching mode, the controller sets the contribution of each actuator as a percentage of the total desired force, as measured by the load cells. In the load-control mode, each actuator retracts and extends to maintain its relative percentage of the total load, reacting to changes in line excursion as the arm is elevated and rotated by the user. The positions of the actuators are recorded continuously and written to a trajectory file defining the desired motion path. To measure the applied forces versus spatial position, the system is set to position-control mode where the actuators replay the excursions recorded in the trajectory file, elevating and rotating the arm based on actuator position.
Optical tracking diode arrays (Optotrak 3020, Northern Digital Inc, Waterloo, ON, Canada) mounted to the distal humerus and ulna anchors monitor the spatial position of the arm as a function of actuator excursion. The spatial arrays are fit to a sphere to calculate the humeral center of rotation using least-squares minimization. Additional Optotrak arrays are mounted to the simulator frame and the embedding block to verify that the structural components of the simulator are rigid. Three digitized points are collected from each surface of the embedding block so data from specimens can be transformed back to the respective CT coordinate systems. This allows the center of rotation of both native and implanted arms to be referenced to the glenoid and scapular plane without exposing the glenohumeral joint.
Question #1—Which Combinations of Joint Tension and Humeral Version in rTSA Optimize Kinematic and Kinetic Outcomes? [31]
Successful outcomes after rTSA ideally maximize range of motion while minimizing instability and deltoid stress. Despite the increasing prevalence of rTSA over the past 10 years, there is still debate on the optimal hardware configuration and placement to achieve these objectives. Optimal humeral version is debated, with authors recommending anywhere between 0° and 30° of retroversion [33–35]. Boileau et al. [6] reported that intraoperative determination of deltoid tension is mostly guided by surgeon experience. Regarding joint tension, placing the implant as tight as possible may minimize the chances of dislocation, but this could be a source of residual deltoid and coracobrachialis/biceps short head-related pain and possibly a risk for acromial stress fractures.
Few biomechanical studies have evaluated the effects of humeral version on outcomes after rTSA [8, 29, 36, 37]. Stephenson et al. [37] evaluated how changes in humeral version affect IR and ER range of motion and determined that 20°–40° of humeral retroversion most closely restores a functional arc of motion without impingement. Berhouet et al. [36] determined that placement of the humeral component in 10°–20° of retroversion (replicating normal version) most effectively limits inferior scapular impingement. Favre et al. [8] evaluated the effect of humeral version on stability and determined that in the setting of neutral glenosphere version, stability increases with humeral version from 0° to anteversion. Finally, Gulotta et al. [29] determined that changes in humeral version had no effect on deltoid force required for scaption, and increased retroversion improved ER but restricted IR. Reports regarding joint tension in rTSA are severely lacking, in part due to the experimental removal or omission of soft tissue structures in the glenohumeral joint.
Our group evaluated the combined effects of changes in joint tension and humeral version utilizing the simulator. We tested cadavers by first evaluating their baseline native range of motion and motion/force profiles for abduction and rotation. We then implanted a Tornier Aequalis Reversed Shoulder prosthesis in each arm. The baseline configuration consisted of 10° of humeral retroversion with a 9 mm polymer insert. The version and tension were then incremented to test all 9 combinations of 0°, 10°, and 20° retroversion with 6 mm, 9 mm, and 12 mm polymer inserts.
The results showed that the humeral center of rotation shifted medially 17 mm and inferiorly 12 mm after implantation of the rTSA, in agreement with prior studies of Grammont-style rTSA [27, 38, 39]. Changes in retroversion had no effect on ER range of motion or stability, but 10° of retroversion resulted in the largest adduction deficit. Increasing polymer insert thickness reduced both abduction and adduction range of motion (Fig. 9.2) [31]. Despite the lost motion, increased implant thickness and the resultant joint tension improved implant stability with no overall increase in deltoid force required for abduction (Fig. 9.3). All implant configurations resulted in a net mechanical advantage, reducing the forces required for elevation in comparison with the native shoulder.
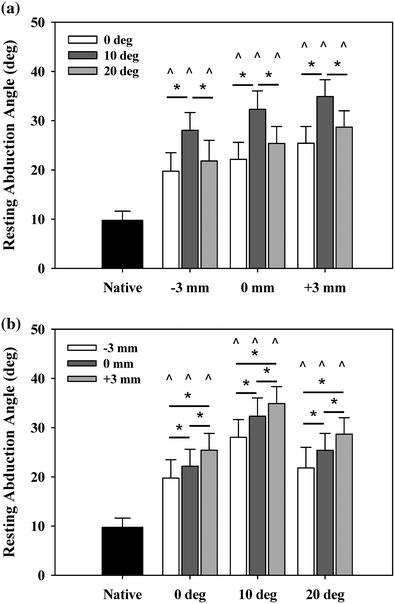
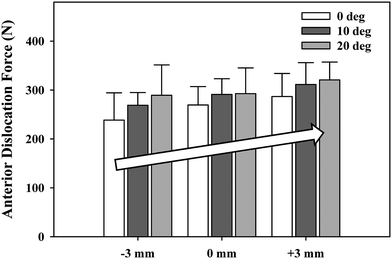

Fig. 9.2
Resting abduction angle. Reverse arthroplasty resulted in significantly increased resting abduction angles (adduction deficit) versus native arms (^). Resting abduction increased a minimum of 10° versus native. a As a function of insert thickness, 10° retroversion resulted in the highest resting abduction. b As a function of version, incremental increases in insert thickness resulted in incremental increases in resting abduction. Mean ± SEM [31]

Fig. 9.3
Increasing poly thickness in the Tornier Aequalis Reversed Shoulder prosthesis significantly increased dislocation force with implant thickness. Lateral dislocation force exhibited a stepwise increase with increasing poly thickness and was significant for all combinations tested (*) [31]
Placing an rTSA between 0° and 20° of retroversion had no effect on rotational range of motion. These findings differ from those of Stephenson and Gulotta who reported changes in retroversion to have an effect on impingement-free range of motion [17, 40]. The results of the prior studies and the current data may differ due to soft tissue tension limiting overall rotation before direct impingement is encountered. Neither Stephenson (cadavers devoid of soft tissue) nor Gulotta (computational model) used full cadaveric shoulders with all soft tissues intact [17, 40]. In our study, increasing implant poly thickness, or joint tension, improved implant stability at the cost of reducing overall abduction/adduction range of motion. While increasing poly thickness limited instability, caution should be exercised since excessive tension was associated with lost range of motion in elevation. In addition, excessive joint tension could exacerbate residual deltoid pain or increase the risk for acromial fracture in clinical populations.
Question #2—What Is the Effect of Isolated Glenosphere Lateralization in a Grammont-Style rTSA? [30]
Two of the major limitations with Grammont-style rTSA are scapular notching and the failure to restore ER, despite significant improvements in forward elevation and abduction. Boileau et al. [6] determined that ER only improved by approximately 5° with a Tornier Aequalis Reversed Shoulder prosthesis where the glenosphere medialized the center of rotation to the glenoid face. In the same study, the authors reported a 68 % scapular notching rate with 7 % dislocation. In 2011, Boileau et al. [5] reported on 42 shoulders that underwent the same rTSA with the addition of a 10-mm bone graft under the baseplate, effectively lateralizing the glenosphere center of rotation. In this follow-up, they reported a 10° improvement in ER, a 19 % scapular notching rate, and no dislocations. These studies suggest that lateralizing the glenosphere with a bone spacer can improve joint stability and reduce notching similar to lateralizing the center of rotation with a lateral offset glenosphere, as available in the Reverse Shoulder Prothesis (DJO, Austin, TX) system [7, 33]. Highlighting these clinical advantages, no laboratory studies previously investigated the biomechanical effects of isolated glenosphere lateralization in a Grammont-style implant with soft-tissue-constrained shoulders.
We examined the effects of glenosphere lateralization using the shoulder simulator. Specifically, incremental metal spacers were created to simulate a bone graft placed under the glenoid baseplate of an rTSA. We tested cadavers by first evaluating their baseline native range of motion and motion/force profiles for abduction. We then implanted a Tornier Aequalis Reversed Shoulder Prosthesis in each arm. The baseline configuration consisted of 10° of humeral retroversion with a 9 mm polymer insert, with the glenosphere flush to the glenoid baseplate. We then progressively lateralized the glenosphere using 5, 10, and 15 mm spacers (Fig. 9.4). Our results showed that lateralization had no effect on passive ab/adduction or IR/ER. Lateralization significantly increased the deltoid force required for abduction in a linear fashion with each 5 mm offset (Fig. 9.5). Lateralization also increased the force to dislocate the shoulder once 10 mm of lateralization was achieved (Fig. 9.6).
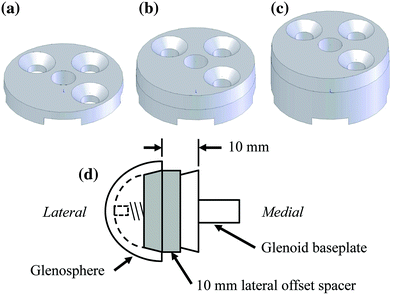
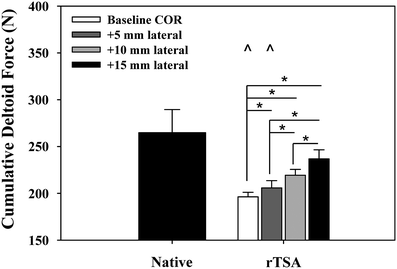
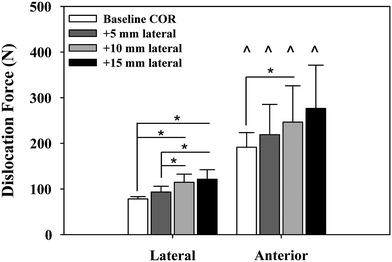

Fig. 9.4
Schematic of custom lateral a 5 mm, b 10 mm, and c 15 mm offset spacers. d Representation shows assembly with a 10 mm offset spacer in place between the fixed glenoid baseplate and the lateral offset glenosphere [30]

Fig. 9.5
Cumulative deltoid force at 60° scapular plane abduction. Reverse total shoulder arthroplasty (rTSA) significantly reduced the force required to abduct the arm for baseline and 5 mm center of rotation offset cases versus the native arms (^). Lateral offset resulted in a stepwise increase in abduction force, which was significant for all cases (*). Mean ± SEM [30]

Fig. 9.6
Force required to dislocate the rTSA laterally (in neutral external rotation) and anteriorly (in 90° external rotation). Lateral dislocation required significantly less force than anterior dislocation for all lateral offset center of rotation cases (^). Lateral dislocation force exhibited a stepwise increase with lateral offset COR, which was significant for most combinations tested (*). Anterior dislocation exhibited a similar trend but showed limited statistical significance (*). Mean ± SD [30]
These data support that glenosphere lateralization is a reasonable method to improve stability, but at the cost of increased deltoid forces to elevate the arm. Lateralization had minimal effect on rotational motion, which again may be attributed to soft tissue tension limiting impingement-free range of motion seen in computational or bone-only models. This data agree well with clinical data reporting improved stability with minimal effect on rotational range of motion [5]. What has not been studied is the potential cost of increased deltoid force requirements on implant longevity. Increased deltoid forces may lead to deltoid-related pain and/or accelerate the functional decline of the implant that has been reported to occur approximately 6 years after surgery [41].
Question #3—Are There Significant Kinematic and Kinetic Differences Between Baseline rTSA Systems Designed Around a Medial or Lateral Center of Rotation? [32]
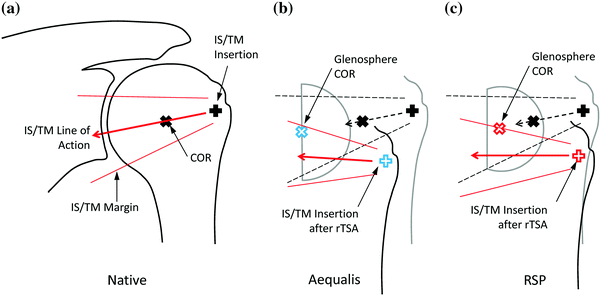
Numerous rTSA systems are available for surgeon use. Early rTSA systems included a lateral offset glenosphere to maintain the anatomic joint center of rotation, but these designs suffered early implant loosening and were abandoned [42, 43]. The Grammont-style implant places the glenosphere on the face of the glenoid to reduce rotational bending moments and shear stress on the glenoid, solving the loosening issue but resulting in scapular notching [40, 44]. Recent rTSA designs have reintroduced a lateralized center of rotation offset, along with improved glenoid fixation, to limit inferior impingement and notching as well as glenosphere loosening seen with earlier lateral designs [7]. Currently, all rTSA systems available to orthopedic surgeons are built around these two primary system designs—lateral and medial center of rotation glenospheres.
Many bone surrogate, computational, and simulator studies have been performed to define the effects of individual design characteristics of rTSA. Despite this extensive literature showing that rTSA configurations can individually affect outcomes, no study has directly compared the composite performance of rTSA systems, including all the design differences unique to each system, in a soft-tissue-constrained cadaver model.
Our group evaluated the performance of two commonly used rTSA systems: the Aequalis Reversed Shoulder (Tornier, Edina, MN, USA) and the Reverse Shoulder Prosthesis (DJO Surgical, Austin, TX, USA). The Aequalis represented a medialized glenosphere Grammont-style system, whereas the RSP represented a lateralized glenosphere system. We examined these systems using the shoulder simulator in 14 fresh-frozen, paired upper extremities. The cadavers were tested by first evaluating their baseline native range of motion and motion/force profiles for abduction and passive rotational ranges of motion. At random, one shoulder of the pair received the Aequalis and the opposing shoulder received the RSP. The most commonly used configuration of each implant was used for each system. The Aequalis Reversed Shoulder was placed in 10° of humeral retroversion with a 9 mm polymer insert, with a 36-mm glenosphere in the 10 degree tilt configuration simulating a 4 mm lateral offset COR. The glenoid baseplate was aligned with the inferior aspect of the glenoid and placed flush on the glenoid face. The RSP was placed in 30° of humeral retroversion with a 32-4 glenosphere in 10° inferior tilt. A neutral humeral shell with a standard polymer insert was used, and the glenoid baseplate was centered on the glenoid face. The manufacturer of each implant supplied data supporting that these configurations accounted for almost 70 % of all implanted cases in the USA and are therefore considered the “most common.”
Both systems shifted the joint center of rotation medially and inferiorly compared to the native shoulder, where the Aequalis had greater shifts than the RSP in both directions. Both rTSA systems lengthened the humerus with respect to native by approximately 23 mm [17]. The medialized Aequalis center of rotation resulted in a medially shifted humerus, whereas the RSP only shifted the humerus inferiorly (Fig. 9.7). Regarding passive ROM (ab/adduction and IR/ER), the RSP resulted in less adduction deficit than the Aequalis (Fig. 9.8), but otherwise, there were no differences between systems. Finally, there were no differences in deltoid force required for abduction between systems, although the force gradient required to initiate abduction was larger for the Aequalis than the RSP (Fig. 9.9). This suggests that it is more difficult to initiate abduction with the Aequalis than the RSP, but both systems provided a mechanical advantage versus the native shoulder.