4

Rotator Cuff Disease and Impingement
Rotator cuff disease is the most common condition of the shoulder for which patients seek treatment. The symptoms of rotator cuff disease increase with age almost linearly, so most people will have one episode of shoulder pain during their adult lives that will be due to rotator cuff problems. Rotator cuff disease also affects athletes and active individuals regardless of age and activity level, and this underscores that there are several possible etiologies of rotator cuff problems.
Exactly what causes rotator cuff problems has been debated for several centuries, and the debate is far from over. New knowledge has begun to question old dogma and to create new theories of why rotator cuff disease occurs and how it produces symptoms. Consequently, a review of the history of the concepts of rotator cuff disease serves to illustrate that the exact etiology of this condition is not known. Knowledge of the pathophysiology of rotator cuff disease is essential to understanding the variability of the complaints of the patient and why the examination continues to be challenging for this common condition.
There is increasing evidence that rotator cuff problems are a multifactorial disease. The most accepted theory in the past 40 years has been on of “impingement,” as popularized by Neer.1 There is increasing evidence, however, that rotator cuff disease may not be due to impingement alone. There is also some evidence that impingement between structures may occur in different ways and in different areas than previously understood. The anatomy of the rotator cuff was described in Chapter 3.
Background
Who first used the term rotator cuff is not obvious in the literature. The first description of its rupture, however, is by Smith in 1834.2 He described seven cases of tears in the rotator cuff in specimens obtained by grave robbing. He described many of the variations of rotator cuff pathology recognized today, ranging from small tears involving the subscapularis to massive degenerative tears with an associated biceps tendon dislocation.
Codman3 revolutionized the knowledge about the musculotendinous cuff, or rotator cuff. In his book, he summarized his 25 years’ experience with rotator cuff disease. He recommended early operative repair for complete cuff tears and is credited with the first cuff repair, in 1909. Other authors noted that the lesions of the rotator cuff seemed to be degenerative with age, but the exact etiology was uncertain.
Theories of Rotator Cuff Disease
There are several current theories regarding the etiology of rotator cuff pathology. These include degeneration of the tendon due to senescence, tearing of an avascular area of the tendons, impingement, tears due to anterior shoulder instability, tears due to trauma, and tears due to superior instability associated with superior labrum anterior to posterior (SLAP) lesions. Some physicians would suggest that tears of the rotator cuff may be due to any of these or to several mechanisms at once. It is likely that some of these mechanisms are associated or related to each other, such that one cause is linked closely to another. For example, Pettersson4 suggested that normal tendons rarely tear due to traumatic mechanisms, whereas tendons that have been affected by age are weaker (and thereby abnormal) and subject to tearing more easily with sudden stress.
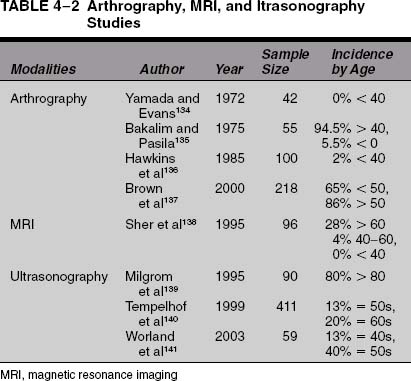
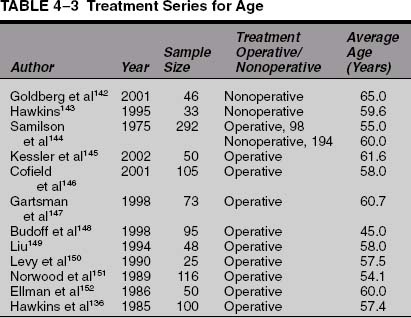
There is significant evidence that rotator cuff tears are due in part to senescence of the tendons with age.1,3,5–11 There is no doubt that the incidence of rotator cuff pathology and tears increases with age. This is supported by cadaveric studies, radiological imaging studies, and patient case series. The cadaveric studies are summarized in Table 4-1. Imaging studies using arthrography, ultrasound, and magnetic resonance imaging (MRI) that support this idea are summarized in Table 4-2. Patient case series that evaluate nonoperative and operative treatment of patients with rotator cuff disease support the contention that rotator cuff disease becomes more symptomatic and more severe with age and are summarized in Table 4-3.

The exact mechanism to explain why aging is associated with tendon tearing is unknown, but there are several theories. The first is a cellular mechanism whereby the fibroblasts undergo senescence and apoptosis with aging.12–14 The fibroblasts are the cells responsible for maintaining the matrix and collagen structure of the rotator cuff tendons. The factors that cause the cells to die over time are unknown, but models of tendon degeneration suggest that early changes are characterized by granularity and a loss of the normal clear wavy outlines of the collagen fibers and bundle of fibers. The connective tissue cells become distorted, and parallelism of the fibers is lost. The cell nuclei become distorted in appearance. Some areas of tendon have a gelatinous or edematous appearance, with loosening of fibers that contain broken frayed elements separated by a pale-staining homogeneous material.15
Another way that the tendon may degenerate over time is due to the tensile and compressive forces that cause shear in the tendon. This is supported in part by evolutionary study of the shoulder joint but also by biomechanical studies of stress and strain patterns within the rotator cuff tendons. The upper extremity of hominids was initially designed for swinging from trees, so the basic conformation of the joint and the surrounding structures was designed more for distractive forces than for compressive forces. Once hominids began to bear weight on the extremity, the joint began to see compressive forces for which it was not optimally designed. As a result, one anthropologist, Dr. Lee Berger (personal communication, 2000) has suggested that shoulder joints in hominids fail in compression, not tension.
This theory is supported by the observation that the rotator cuff muscles function the most to provide a compressive force on the joint when it is used for lifting objects. Electromyogram studies have shown that the rotator cuff muscles fire the most when lifting a weight away from the body, which is when the lever arm is between the shoulder and the object is the longest.16,17 This also is supported in part by the complaint of patients with a symptomatic rotator cuff tear that their pain is the worst when they reach to pick up an object away from their body, such as a gallon of milk in the back of the refrigerator. It would also explain why patients with rotator cuff pathology have more pain bringing the arm down from an elevated position than they do elevating it: there is more compressive force on the way down than there is on the way up.
Biomechanical studies of the rotator cuff support the idea that the rotator cuff is subjected to high compressive and tensile forces that may contribute to pathology. Soslowsky et al,18 who utilized MRI in a rat model, demonstrated a similar pattern. The results of this study demonstrate that the injury created by overuse plus extrinsic compression is greater than the injuries created by overuse or extrinsic compression individually. Without an additional factor, such as overhead activity, the extrinsic compression alone may be insufficient to cause tendinosis.
Luo et al19 suggest that the tearing of the tendon may occur in an area where the compressive and tensile forces create shear in the tendon. They studied a two-dimensional finite element model, and subacromial impingement was simulated by producing indentation on the bursal surface of the supraspinatus tendon. The researchers found that subacromial impingement generates high stress concentrations and that such stress could initiate a tear; however, these tears can occur on the bursal side, the articular side, or within the tendon.
These studies may explain in part the suggestion that rotator cuff failure occurs in overhead athletes as a result of a “tension overload.”20–22 Studies using motion analysis demonstrate that there are several large forces upon the shoulder joint in the overhead throwing motion of baseball pitchers. The first is an anteriorly directed force at the time of ball release.23 This force has been reported to be of a magnitude ~310 N, depending on the population studied and the techniques used in the particular study. The second large force is posteriorly directed during the follow-through phase and was found to be 1090 N.23
The largest forces seen on the shoulder joint complex, however, are at the time of ball release, when the arm is essentially pulled away from the body23–25 (Fig. 4-1). This force has been reported to be between 900 and 1100 N, depending on the study population.23 This distractive force has to be resisted by the ligaments and tendons of the shoulder joint, which create a compressive force to prevent distraction of the arm. One theory is that this force is also resisted by the rotator cuff muscles; once the muscles fatigue, the ligaments and tendons cannot provide the necessary compressive force, and they begin to stretch. This failure allows the joint to become looser and the ligaments more lax; eventually, other structures begin to fail, such as the labrum and the rotator cuff tendons. Although it is a distractive force on the joint that initiates the failure, the joint fails by not providing the compressive forces necessary to protect the integrity of the joint.
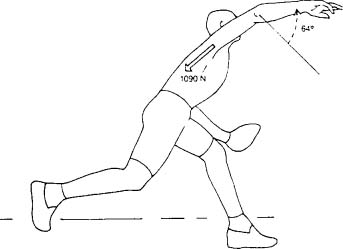
FIGURE 4-1 The largest forces on the arm during the baseball throw are compressive forces that resist the arm being distracted off the shoulder. (Adapted with permission from Fleisig G, et al. Kinetics of baseball pitching with implications about injury mechanisms. AJSM 23(2):233–239, Fig. 7.)
This theory is part of the model of cuff tearing suggested by Morgan et al,26 who postulated that the initiating event for rotator cuff tearing is repetitive twisting and torquing of the biceps anchor with overhead or throwing motions. According to their theory, with the arm in abduction and external rotation, the biceps anchor is twisted such that it causes the superior labrum attachments to eventually tear. This labrum lesion is one of three types of type II SLAP lesions (see Chapter 6). Once the labrum tears, it creates a “superior instability” pattern, where there is increased stress on the rotator cuff. With throwing, the tensile stress on the cuff increases, and the tendon eventually tears. Morgan et al suggest that this superior instability pattern can be corrected by repairing the SLAP lesion. In some instances the ligaments (specifically the anterior band of the anteroinferior glenohumeral ligament) may become stretched due to this superior instability pattern, and this anteroinferior instability pattern may need to be treated with some capsular tightening procedure. The options include an open capsular shift,27 a thermal capsular shift,28,29 or an arthroscopic capsular shift.29
Another theory is that the pain of rotator cuff disease is actually due to a pain syndrome and not inherently due to the tearing of the tendon alone. According to this theory, the nerve endings of the bursa become activated by cytokines produced when the tendon cells see too much stress. Once these fibers become sensitized, the pain continues until the cycle is broken by nonoperative or operative methods. This would explain why injections into the subacromial space relieve shoulder pain, and it may explain why some patients get relief from their tendinitis pain when only local anesthetic is injected into the subacromial space.
Alvarez et al30 injected 28 patients with xylocaine as a control group and 30 patients with xylocaine and corticosteroid. They found that, although both groups showed improvement in disease-specific quality of life, there was no statistically significant difference between the betamethasone group and the Xylocaine group and the Xylocaine alone group. Other evidence to support the relationship of cytokines to shoulder pain is from the cell biology studies of Gotoh et al,31 who proposed that the differential regulation of the two forms of interleukin (IL)-1ra in synovial cells in the subacromial bursa may play an important role in shoulder pain in patients with rotator cuff disease.
The fourth most accepted theory of rotator cuff disease is that the rotator cuff tendons tear by impinging upon other structures. Although this has been the commonly accepted theory of cuff disease since the mid-20th century, there is increasing evidence that the etiology of rotator cuff problems is more complex than just one structure impinging upon another. This theory will be evaluated in more detail in the next section.
Types of Impingement
The history of the concept that the rotator cuff impinges upon the rotator cuff was discussed earlier in this chapter. Neer1 suggested that the supraspinatus tendon made contact with coracoacromial ligament and acromion, and this was a major factor in the development of rotator cuff tears (Fig. 4-2). These two structures comprise the “outlet” of the subacromial space. The observations that support the idea of outlet impingement include the presence of erosions of the greater tuberosity, eburnation of the acromion, and spurs in the coracoacromial ligament and acromion.3,32–38 Anatomic studies verify that these changes increase with age and that they are more prevalent in patients with rotator cuff pathology.1,37,39 Neer also suggested that the rotator cuff may impinge upon osteophytes under the acromioclavicular (AC) joint.1,10 The increased arthrosis of the AC joint over time may explain increased tearing of the rotator cuff should this abrasion of the cuff occur.38,40,41
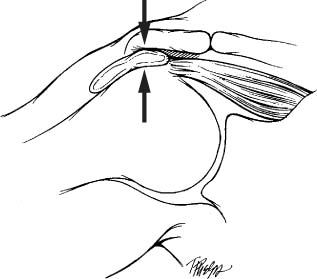
FIGURE 4-2 The theory of subacromial impingment suggests that rotator cuff tearing is due to the supraspinatus tendon impinging upon the “outlet.”
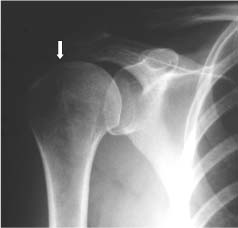
FIGURE 4-3 Normal anteroposterior radiograph of the shoulder demonstrating a normal distance between the acromion and the humeral head of around 1 cm (arrow).
Radiographic studies have been used to support the idea of subacromial impingment. Plain radiographs demonstrate changes associated with rotator cuff disease, and these include erosions of the greater tuberosity, eburnation of the acromion, and spurs in the anterior acromion and coracoacromial ligament.5,42–45 On the anteroposterior (AP) view, the distance between the humeral head and the acromion can be a hint that there is a rotator cuff tear (Fig. 4-3). This distance is normally 7 to 14 mm, but if it is below 7 mm, there is a suggestion of rotator cuff tearing (Fig. 4-4).44 If that distance is below 5 mm, there is a significant chance of a full-thickness rotator cuff tear44; however, not all of the patients with rotator cuff tears had narrowing below 5 mm, so a normal humeral head-acromial distance does not rule out a rotator cuff tear. If there is no distance between these two structures, then the presence of a massive tear should be entertained.
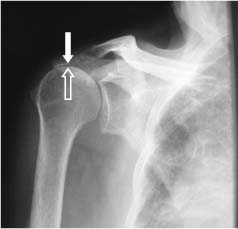
FIGURE 4-4 An anteroposterior radiograph of the shoulder demonstrating a decreased space between the humeral head (open arrow) and the acromion (solid arrow). When there is less than 5 millimeters of space between these two structures, a rotator cuff tear is confirmed.
Leclercq46 has described a dynamic or stress view to determine if the subacromial space between the humeral head and acromion is diminished. Because the AP view with the arm dependent may not close down the space, the AP view is obtained with the arm abducted to 45 degrees against resistance. Abduction of 10 to 20 degrees is preferable to prevent the greater tuberosity from sliding under the coracoacromial arch. With muscle force on the shoulder, specifically the deltoid and rotator cuff, the space will be more accurately assessed, and the contact between acromion and humeral head indicates the presence of a large tear.45
A Grashey view or true AP view of the shoulder is helpful for demonstrating the presence of glenohumeral arthritis, including narrowing of the glenohumeral joint and spurs on the inferior margin of the humeral head.47 This view is helpful when trying to define the degree of arthritis present if there is a known rotator cuff tear, or if there is a question if the pain is due in part to arthritis of the joint.
The presence of an anterior acromial spur (Fig. 4-5) has been associated with rotator cuff disease. A modified AP radiograph was recommended by Rockwood and Jensen48 to visualize an anterior acromial spur better. To our knowledge the clinical utility and accuracy of this view have not been studied.
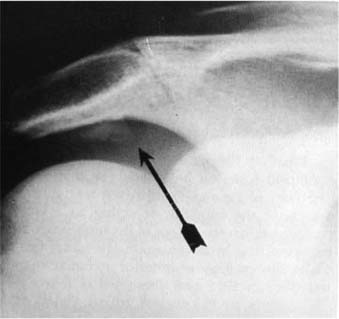
FIGURE 4-5 Radiographs can detect the presence of a spur on the anterior acromion (arrow), but the need to remove this spur surgically is now being questioned.
The shape of the acromion has been associated with the development of rotator cuff disease. In 1986 Bigliani et al49 studied the shape of the acromion in 140 cadavers and its relationship to rotator cuff tears. The average age was 74.4 years. They found that overall incidence of full-thickness tears was 34%. Three types of acromion were identified: type I, which was flat, was found in 17%; type II, which was curved, in 43%; and type III, which was hooked, in 39% (Fig. 4-6). The type III acromion was present in 70% of the rotator cuff tears, whereas only 3% of type I acromions were associated with a tear. In addition, anterior acromial spurs were noted in 14% of the series overall, but 70% were present in patients with rotator cuff tears. Morrison and Bigliani50 applied this concept to a clinical population using a supraspinatus outlet view. In this series of 200 consecutive patients with various shoulder complaints, they found that 35 patients had a full-thickness rotator cuff tear, and 70% had a type III acromion. It should be noted, however, that their study did not delineate definitive criteria for each acromial shape. Although they proved an association between acromial shape and rotator cuff pathology, they did not establish causality.
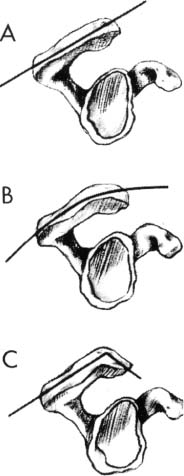
FIGURE 4-6 Three acromial shapes have been described, but it has been debated whether they are a cause or an effect of rotator cuff disease, and whether they are a normal part of aging. (Adapted with permission from Bright A, et. al. Reliability of radiographic evaluation for acromial morphology. Skeletal Radiol 1997;26:718, Fig. 1.)
These criticisms were to form the basis of a controversy that continues to the present day. A study by Bright et al51 evaluated the inter- and intraobserver reliability of Bigliani et al’s classification system. The supraspinatus outlet views of 40 patients were reviewed twice, 4 months apart, by six reviewers. For each of the two readings, all observers agreed only 18% of the time, and Bright et al suggested that more definitive criteria are needed to distinguish and classify the acromion. A study by Jacobson et al52 reported similar results.
Several subsequent researchers attempted to duplicate the findings of Morrison and Bigliani.50 Aoki et al53 developed a technique of measuring the slope of the acromion with a lateral radiograph in the plane of the scapula. Their investigations of bleached skeleton shoulders revealed that the acromial spur and narrowing of the supraspinatus outlet are associated with a flatter acromial slope. This supported Neer’s original statement implicating the increasing slope of the acromion in the impingement syndrome.1 Edelson and Taitz54 found that the length of the acromion plays an important role in the development of degenerative changes and associated cuff problems. Nicholson et al55 demonstrated on a review of 420 scapulae that spur formation of the anterior acromion was an age-related process such that individuals younger than 50 years of age had less than one quarter the prevalence of those older than 50 years of age. The status of the rotator cuffs of these shoulders was unknown.
Ozaki et al7 studied the histology of the acromial undersurface in 200 cadavers and found that rotator cuff tears that did not extend to the bursal surface were associated with normal acromial histology, whereas those that extended to the bursal surface were associated with pathologic changes. Fukuda et al56 also concluded that most cuff tears are related to tendon degeneration and that acromial changes are secondary to pathology of the bursal side of the cuff. Although the above studies indicate a strong association between aging and the presence of cuff tears, it is unclear whether the changes in acromial morphology were caused by or resulted from the cuff defects, or whether both were the consequences of an aging rotator cuff.
Studies of acromial shape support the concept that acromial spurs are not the only factor in the development of rotator cuff disease and that the change of acromial shape may be a result of shoulder stress.
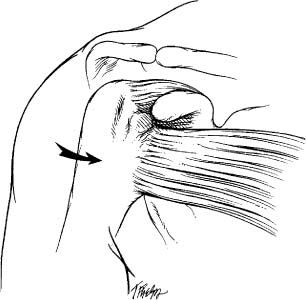
FIGURE 4-7 With the arm in flexion and internal rotation, the lesser tuberosity and bicipital groove (hatched area) come into contact with the acromion and coracoacromial arch.
Coracoid Impingement
Coracoid impingement as a source of shoulder pain was first described by Goldthwait in 1909.57 He suggested that the subcoracoid bursa could be irritated by compression between the coracoid and the humeral head (Fig. 4-7). He also noticed that the size and shape of the coracoid may be an explanation of why the impingement occurred in some individuals and not in others; however, Codman did not agree with this concept, and it did not receive much attention in the literature for many years.
Gerber et al58 can be credited with resurrecting the concept that the coracoid may be a source of contact between the rotator cuff and the coracoid as a source of pain. They called this entity “subcoracoid impingement,” because they felt that the subcoracoid space was bounded by the acromion superiorly, the coracoid anteriorly, and the coracoacromial ligament anterolaterally. They noticed that patients who had anterior shoulder pain had a previous surgical procedure for instability known as a Trillat procedure. In a Trillat procedure, the coracoid is osteotomized and rotated so that it juts out in front of the joint. This operation had as its rationale the idea that the bone would prevent anterior instability of the shoulder by acting as a bony block. In some patients who had this operation, the researchers identified a loss of internal rotation, tenderness over the tip of the coracoid, and anterior shoulder pain that could radiate down the arm.
Gerber el al58 suggested that the pain of coracoid impingement often could radiate such that it would have the appearance of a cervical radiculopathy. These patients typically would have a negative Neer impingement sign in flexion, suggesting that the impingement did not occur in flexion because the humeral head could clear the coracoid; however, placing the arm in a Kennedy-Hawkins impingement position (forward flexion to 90 degrees and internal rotation) was found to reproduce the pain in many patients.
Gerber et al58 and Dines et al59 suggested that injection of local anesthetic into the subcoracoid space may help in making the diagnosis of coracoid impingement. Gerber et al58 found that injecting the subacromial bursa also may be necessary to distinguish between coracoid impingement and subacromial impingement, but they did not report upon the accuracy of the injections or upon the clinical effectiveness of these injections in making the diagnosis. They also suggested that despite computed tomography (CT) scanning, sometimes the diagnosis could not be definitely made until the time of surgery, when the arm could be placed into internal rotation and flexion, demonstrating impingement of the structures to the coracoid.
Gerber et al58 felt that there were several possible clinical scenarios where coracoid impingement may occur; these included idiopathic, iatrogenic, and posttraumatic forms. The first, or idiopathic, form was found in individuals with no previous injuries or surgeries where the condition was felt to be due to a prominent coracoid process. In their series of 37 shoulders, 4 were felt to have the idiopathic form. In those patients the diagnosis was made upon clinical examination and confirmed with CT scanning. In one of these patients, isolated removal of the tip of the coracoid relieved the pain and produced a normal shoulder. In the other three patients the tip of the coracoid and the coracoacromial ligament were removed, and two had a standard anterior acromioplasty.
Dines et al59 reported on eight patients with symptoms of coracoid impingement; four had an idiopathic form. In their series the patients’ pain was improved with osteotomy of the coracoid, removal of the tip, or removal of the outer 1.5 cm of the coracoid. Ferrick60 reported on one patient with idiopathic coracoid impingement who did well with only 6 months of follow-up after distal coracoid excision. He concluded that subcoracoid impingement was uncommon but should be considered in patients who have anterior shoulder pain, especially if the pain is not relieved with treatment of subacromial impingement.
In a subsequent study, Gerber et al61 found that in normal volunteers the distance between the coracoid and the humeral head depended on the rotation of the humeral head. They found that with the arm at the side, the humeral head was closest to the coracoid when the arm was in internal rotation (8.7 ± 2.4 mm) versus neutral or external rotation. Next, Gerber et al studied the shoulders using CT with the arm in a position simulating coracoid impingement: flexed (90 to 100 degrees), horizontally forward flexed (90 to 115 degrees), and fully internally rotated. They found that the distance between the humeral head and the coracoid decreased to 6.8 ± 2.9 mm (range 2.5 to 13 mm) with the arm in this position; this was statistically different from the distance when the arm was at the side. They also described other measures of the relationship between the glenoid, coracoid, and proximal humerus that may further delineate the relationships that would predispose an individual to coracoid impingement.
Friedman et al62 subsequently measured the coracohumeral distance with the arm in maximal internal rotation using MRI in symptomatic and unsymptomatic shoulders. They found that in normal shoulders the coracohumeral distance average was 11 mm (range 4 to 17 mm), whereas in symptomatic patients it was 5.5 mm (range 0 to 1 mm).
Both Gerber et al58 and Patte63 emphasized that bony impingement between the humerus and the coracoid could not be demonstrated due to the interposition of soft tissue in the subcoracoid space. These structures in the subcoracoid space included the subscapularis tendon; the joint capsule, including the superior and middle glenohumeral ligaments; the articular cartilage of the humeral head; and the biceps tendon. Valadie et al64 studied cadavers with the shoulder in flexion and internal rotation, such as seen with a Kennedy-Hawkins sign. They found that the subscapularis tendon was deformed by the coracoid in one of four specimens.
The role of the subscapularis in filling the subcoracoid space was not supported by a study by Nove-Josserand et al,65 who measured the coracohumeral distance using CT scanning in 206 patients with rotator cuff tears. They found that the distance was the same in patients with an isolated subscapularis tear (9 mm) as it was in patients with tears of the supraspinatus and infraspinatus (9 ± 2 mm); however, the coracohumeral distance was statistically smaller in patients who had combined tears of the infraspinatus, supraspinatus, and subscapularis. Nove-Josserand et al concluded that the coracoid probably was not a factor in isolated subscapularis tendon tears. They also suggested that a tear of the subscapularis tendon was necessary with tears of other tendons to produce narrowing of the coracohumeral head distance.
The second cause of coracoid impingement was felt to be iatrogenic after a Trillat procedure or after posterior glenoid osteotomies. Gerber et al58 suggested that in 56 Trillat procedures that his group had performed, symptoms of coracoid impingement were common. They felt that the humeral head was making contact with the coracoid and that it could cause pain and loosening of the screw used to secure the coracoid in position.
Another cause of iatrogenic coracoid impingement was found by Gerber et al58 in two patients who had previous posterior glenoid osteotomies for posterior shoulder instability. In those patients, the persistent anterior shoulder pain was relieved by coracoidplasty. The researchers speculated that changes in the glenoid tilt may have predisposed the humeral head to abut the coracoid. Dines et al59 reported one case of coracoid impingement due to a posterior glenoid osteotomy. In our personal experience, anterior shoulder pain is a frequent occurrence after posterior capsular shifts for posterior instability; however, all of those patients had resolution of the pain with time, and the exact etiology of the anterior shoulder pain could not be established.
The third cause of coracoid impingement could be posttraumatic, where deformities are created that can produce impingement symptoms. In their58 series of patients, one patient had a proximal humeral malunion after an anterior fracture-dislocation of the shoulder, and a second had malunion after a three-part fracture of the humeral head. Both were successfully treated with a shortening procedure of the coracoid. Another patient who apparently did not undergo surgery had symptoms of coracoid impingement after a proximal humeral rotational osteotomy for persistent anterior shoulder instability. Dines et al59 reported one case of a malunion of a lesser tuberosity fracture that caused coracoid impingement.
Gerber et al58 also suggested that impingement upon the coracoid could occur with processes that might enlarge the greater tuberosity. They reported two cases of calcific deposits that caused impingement upon the coracoid; one was in the supraspinatus tendon. Patte63 suggested that another cause of coracoid impingement could be an increase in the size of the contents of the subcoracoid space. This increase in volume could be due to an isolated tear of the subscapularis tendon with subsequent dislocation of the long head of the biceps tendon, scar formation after tearing of the coracohumeral ligament, or calcification of the subscapularis tendon. He also suggested that laxity due to partial tears of the supraspinatus may lead to a “functional” impingement upon the coracoid. Ko et al66 reported a case of a ganglion cyst arising from the subscapularis tendon as a cause of coracoid impingement.
The exact pathophysiology of coracoid impingement remains unknown, and criteria for making this diagnosis remain unclear.
Internal Impingement
The concept of internal impingement was introduced by Walch et al67 in 1992. They described contact of the rotator cuff to the posterior and superior glenoid when the arm is placed in an abducted and externally rotated position (Fig. 4-8). They noted this contact in patients undergoing diagnostic arthroscopy of the shoulder by placing the arm into this position with the arthroscope in a posterior portal. Their patients included 17 athletes; all but 1 played a throwing sport. None of their patients had signs of anterior shoulder instability, and all had pain posteriorly and superiorly with the arm in abduction and external rotation.
FIGURE 4-8 Internal impingement of the shoulder was initially described during arthroscopic evaluation of the shoulder with the arm in abduction and externally rotated.
At the time of arthroscopy, 14 of the patients had pathology of the rotator cuff, 12 had lesions of the posterosuperior labrum, and 8 had osteochondral lesions higher up on the humeral head than a typical Hill-Sachs lesion. Of the rotator cuff tears, 10 involved partial tears of the supraspinatus, of which three were type I Ellman tears and the rest were type II. Walch et al67 classified the partial-thickness cuff tears according to the Ellman classification,68 where the normal tendon is considered 10 to 12 mm thick. According to this classification, a grade I partial tear is less than 3 mm deep. A grade 2 lesion is 3 to 6 mm deep but does not exceed one half of the thickness of the tendon. Lesions more than 6 mm in depth are grade 3 partial cuff tears (Table 4-4). These tears can be on the articular surface, on the bursal surface, or within the tendon. The tears within the tendon are not visible from the surface and occur in the interstitial layers of the tendon. In addition, three tears involved the infraspinatus (of which two were type I Ellman tears and one was type II).
Walch et al67 suggested that this was a new type of impingement that was distinctly different from the Neer impingement and from F. Jobe’s69 theory of instability causing cuff pathology (Fig. 4-9). Although they also suggested that the contact of the rotator cuff to the posterior and superior glenoid that they observed may be physiological (i.e., normal), they postulated that in the throwing or overhead athletes, partial tears of the rotator cuff were due to this repetitive contact of the rotator cuff to the posterior and superior glenoid.
| Location | Grade |
| Partial-thickness tear | I: < 3 mm deep |
| Articular surface | II: 3–6 mm deep |
| Bursal surface | III: > 6 mm deep |
| Interstitial |
Ellman H. Arthroscopic subacromial decompression: analysis of one- to three-year results. Arthroscopy. 1987;3(3):173–81.
FIGURE 4-9 A drawing of the contact of the greater tuberosity of the humerus and of the rotator cuff tendon to the superior glenoid with the arm in abduction and external. This type of contact was originally called internal impingement. (Adapted with permission from Jobe F, ed. Operative Techniques in Upper Extremity Sports Injuries. St. Louis: Mosby, 1996:170, Fig. 7-6B.)
Further evidence of this type of contact was supplied by subsequent anatomic, radiological, and clinical studies. C. Jobe70 reported on cadavers that were fixed in abduction and external rotation and found that the posterosuperior labrum was deformed by the greater tuberosity.
Radiographic studies also demonstrated that this contact could be visualized with MRI arthrography.70,71 Tirman et al71 studied eight competitive athletes (seven baseball players and one volleyball participant) with shoulder pain who were found to have internal impingement arthroscopically using MRI (Fig. 4-10). The researchers concluded that MRI-arthrograms were more sensitive for internal impingement and for detecting undersurface rotator cuff tears and posterosuperior labrum lesions. They found that the infraspinatus tendon was more commonly involved with partial tears in this group than were supraspinatus tears. They suggested that these patients had anterior instability based on the examination under anesthesia, but there were no Bankart or Hill-Sachs lesions found at the time of arthroscopy.
The first report to suggest that internal impingement may be associated with anterior instability was by Liu and Boynton72 in 1993, which described a patient with posterosuperior impingement arthroscopically who had signs of increased laxity and possible anteroinferior instability. Davidson et al73 in 1995 reported on internal impingement in two patients who were professional pitchers with anterosuperior and posterosuperior shoulder pain associated with throwing. They found partial tears of the posterior supraspinatus, posterosuperior labrum lesions, and diminutive Inferior Glenohumeral Ligaments (IGHLs) in both patients.
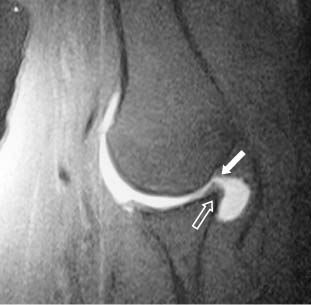
FIGURE 4-10 Internal impingment of the rotator cuff (closed arrow) on the glenoid (open arrow) with the arm in abduction can be demonstrated with magnetic resonance imaging enhanced with gadolinium (oblique coronal view).
Davidson et al73 speculated that the partial cuff tears were due to contact of the rotator cuff to the posterosuperior labrum. They suggested that this phenomenon could occur in patients with no instability, and in these cases “hyperangulation” of the proximal humerus in abduction and external rotation as seen in overhead athletes allowed the greater tuberosity to impact upon the glenoid. The second way this contact could occur was when the IGHL was lax; this laxity would allow increased anterior translation and contact of the greater tuberosity to the posterior and superior glenoid. Davidson et al73 also speculated that scapular malpositioning may contribute to this condition, but it was not specifically studied in their two patients.
This concept of internal impingement was expanded later by C. Jobe,70 who suggested that it may be seen not only in overhead athletes but also in patients whose occupations involved abduction and external rotation of the shoulder. Although Jobe’s series included two greater tuberosity fractures that did not undergo arthroscopy, in the eight patients in the series who underwent arthroscopy, there was damage in seven patients to the rotator cuff, the superior labrum, or the posterior humeral head (i.e., Hill-Sachs lesion or humeral head chondromalacia).
C. Jobe74 suggested with the mechanism of abduction and external rotation that five structures were at risk. These included the posterosuperior labrum, the under-surface of the rotator cuff, the IGHL, the bone of the greater tuberosity, and the bone of the glenoid. The IGHL ligament was not directly at risk due to contact but rather to progressive stretch.73,75 Jobe noted two cases of superior glenoid stress lesions of the superior glenoid tubercle fracture, which were documented in overhead throwing sports by Iannotti and Wang,76 as evidence that the superior glenoid can be at risk with internal impingement. He also speculated that greater tuberosity fractures can occur with the arm in abduction and external rotation when the greater tuberosity comes into contact with the superior glenoid.
Jobe speculated that this contact may be physiological in some individuals, and he speculated that there were several reasons for this contact to become symptomatic. The first reason was due to the repetition of the motion as seen in overhead throwers.70 Fatigue of the rotator cuff muscles may allow increased angulation and contact of the humeral head to the posterior and superior glenoid. Abnormal motions of the scapula, known as scapular dyskinesis, may contribute to internal impingement by allowing increased contact seen in internal impingement. Stretching of the IGHL may increase anterior translation and result in damage to the structures at risk70,73,75,77 In the overhead athlete, the internal impingement progressed to a final stage where the IGHL would have to be surgically shortened to remedy the condition.73,75 Subsequent studies have confirmed the suspicions of Walch et al67 and of C. Jobe70 that contact of the rotator cuff to the posterior and superior glenoid is probably physiological.78–80 These studies have included an anatomic study, a radiological study, and a clinical case series.
A clinical case series was reported by McFarland et al79 of 105 consecutive patients who were undergoing shoulder arthroscopy for a variety of conditions. Eighty-five percent of the patients made contact to the posterior and superior glenoid when the arm was placed in abduction and external rotation. Contact was made at an average of 95 degrees of abduction and 74 degrees of external rotation. There was no relationship between shoulder laxity, a diagnosis of instability, a partial rotator cuff tear, overhead sports, or impingment signs preoperatively. McFarland et al concluded that because this contact was so common regardless of diagnosis, it should be referred to as “internal contact” and not “internal impingement” until definitive criteria for the latter term could be established.
Halbrecht et al78 performed MRI-arthrograms on the throwing and nonthrowing shoulders of 10 collegiate baseball players. Only three of the subjects complained of problems with their shoulders, but all could participate in their sport. All were placed in the MRI in elevation and external rotation. All of the shoulders demonstrated contact of the rotator cuff to the posterior and superior labrum, and the authors concluded that this contact was physiological. They also could not find any relationship between laxity of the shoulder and this contact.
A cadaveric study of 1232 shoulder skeletal bone specimens was performed by Edelson and Teitz.80 They fixed the scapula in a vice and then moved the humerus in different directions to see which portions of the humerus would impinge upon which structures on the scapula with simulated movements. Like many previous investigators, they found that with abduction the greater tuberosity would hit the superior glenoid and that full elevation could not be reached without external rotation of the humerus. They noted that it was not the acromion that prevented motion of the greater tuberosity, but that the glenoid was the obstruction to the humerus elevation. They also suggested that the contact of the acromion was with the shaft of the proximal humerus and not to the greater tuberosity.
The second piece of evidence that Edelson and Teitz80 interpreted as indicative of internal impingement was the presence of an osteophyte on the posterosuperior glenoid in most specimens (Fig. 4-11). They suggested that the pattern of osteophytes on the humeral head and the glenoid were where contact between the two bones occurred. Though provocative, their study did not have any muscle or soft tissue present. In addition, the scapula was fixed in their study, and normal relationships between the two bones as seen clinically were not examined.
In summary, internal contact of the rotator cuff and greater tuberosity to the posterior and superior glenoid are probably physiological. Findings seen in overhead athletes that have been associated with this contact are partial rotator cuff tears (of the posterior supraspinatus or of the anterior infraspinatus, or both) and posterosuperior labrum pathology with either posterior type II or combined anteroposterior type II SLAP lesions (see Chapter 6). There is no clear association of internal contact with anterior instability or with increased laxity, but there is support for the concept that some form of instability or laxity is associated with superior labrum pathology and partial rotator cuff tears seen in overhead athletes. This is discussed further in Chapter 6.
FIGURE 4-11 It has been postulated that an osteophyte on the posterosuperior glenoid rim in a cadaveric study may be evidence of internal impingement as a physiological phenomenon. (Adapted with permission from Edelson G, Teitz C. Internal impingement in the shoulder. J Shoulder Elbow Surg 2000;9(4):308–315, Fig. 7.)
Because there continues to be speculation about the exact role internal contact plays in the development of these lesions (i.e., partial cuff tears and SLAP lesions), recommendations about treatment are varied. C. Jobe et al73,75 recommend physical therapy if the patient has stage I internal impingement syndrome or if there are no lesions intra-articularly upon arthroscopy. Stage I is characterized by pain with throwing and with the arm in abduction and external rotation, but the patient can still throw.75 In Stage II the patient has a positive relocation maneuver for pain (see Chapter 5). For stage III disease, where the patient can no longer perform the activity, debridement of the partial cuff tear, repair of the SLAP lesion, and capsular tightening are recommended.73,75
Riand et al81 at one time recommended a proximal humerus osteotomy to rotate the humeral head so that the contact would not occur; however, the failure rate was high, so the authors have since abandoned that procedure to treat internal impingement. Burkhart et al82 have suggested that internal impingement is secondary to a superior instability pattern that is due to posterior or combined types of type II SLAP lesions. They recommended repairing the SLAP lesion and suggested that anterior pseudolaxity will be alleviated by this procedure alone.
Contact in Flexion
With the discovery of internal contact with the arm in abduction and external rotation, it was only natural that attention be directed toward contact of the rotator cuff when the arm is placed in flexion, such as in a Neer impingement maneuver. C. Jobe was the first to observe this in MRI studies (personal communication, 1997). He noted that in studies of internal contact using MRI, the arm was actually in forward elevation and not abduction due to the constraints of the tunnel of the MRI. He also noted that the rotator cuff made contact with the anterior and superior glenoid with the arm in this position.70
An anatomic study by Valadie et al64 in association with C. Jobe explored this observation further. In this cadaveric study, the arm was fixed using a special fixative foam, and the shoulder was then sectioned in axial, coronal, and sagittal views. The specimen was fixed with the arm in forward elevation similar to a Neer impingement sign. Valadie et al found that the greater tuberosity made contact with the lateral edge of the acromion in three of five specimens, but that the soft tissue of the supraspinatus made contact with the superior glenoid rim in all five specimens (Fig. 4-12). They found no contact of the rotator cuff to the coracoid with the arm in flexion (as in a Neer impingement sign).
A clinical study by Struhl83 of 10 patients he had examined during arthroscopy also demonstrated contact of the undersurface of the rotator cuff with the superior glenoid when the arm was flexed. All of the patients had symptoms of “classic impingement” upon preoperative evaluation, but the exact examinations used were not mentioned. Struhl called this phenomenon anterior internal impingement. He reported that after arthroscopic debridement of the rotator cuff tear, at short-term follow-up (3 to 6 months), six of nine patients were pain free, two had pain with activities, and one was no better.
In another clinical study, Kim and McFarland84 studied 376 consecutive patients undergoing shoulder arthroscopy to document the presence of contact of the rotator cuff with the superior glenoid in flexion. They prospectively evaluated the patients by placing the arm in flexion with the patient under anesthesia and with the arthroscope in a posterior portal. They flexed the arm forward and noted whether contact of the rotator cuff occurred with the superior glenoid or not; if it did occur, they measured with a handheld goniometer the degree of arm flexion where the contact occurred. Seventy-four percent of the patients demonstrated contact of the rotator cuff with the superior glenoid in flexion.
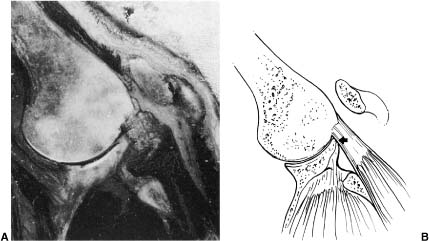
FIGURE 4-12 A cadaveric study of internal impingement with the arm in flexion also demonstrates contact of the rotator cuff (arrow) and greater tuberosity on the anterosuperior glenoid during humeral flexion. (Adapted with permission from Jobe F, ed. Operative Techniques in Upper Extremity Sports Injuries. St. Louis: Mosby; 1996:172, Fig. 7-10A,B.)
In their second study, McFarland et al85 measured the degrees of flexion in patients with a positive Neer sign preoperatively and then measured the degrees of flexion where rotator cuff tendon contact was made with the superior glenoid when viewed arthroscopically. One hundred forty-four patients who had a positive Neer impingement sign were studied. The diagnosis of patients with a positive Neer sign included 23 with instability, 51 with impingement with or without a partial rotator cuff tear, 27 with a full-thickness rotator cuff tear, and 43 with other diagnoses, such as avascular necrosis, loose bodies, and acromioclavicular arthritis. Of these patients who had a positive Neer sign preoperatively, 116 (80%) had a positive Hawkins sign. There were 30 SLAP lesions, with 19 type I, 7 type II, 3 type III, and 1 type IV lesions.
The preoperative arm position in degrees of flexion where pain occurred in patients with a positive Neer sign was 116.7 ± 26.27 degrees (confidence interval 112–121 degrees). There was no statistically significant relationship between the degree of flexion where pain occurred and the diagnosis. There was also no relationship between the degree of flexion where pain occurred and multiple preoperative factors such as sex, age, dominance, sport, and multiple other preoperative and intraoperative findings. During surgery the patients all made contact of the rotator cuff to the superior glenoid at 122.5 ± 18.6 degrees (confidence interval 119–125.6 degrees). The degrees of contact in flexion of the rotator cuff to the superior glenoid were statistically different from the degrees in flexion preoperatively where a Neer impingement sign produced symptoms (p < .05).
Although this study suggested that pain with impingement occurs prior to actual contact of the rotator cuff to the superior glenoid, it is likely that the difference in the two measures was statistically significant, though probably not clinically different. In other words, the goniometric measures indicate that the rotator cuff does become painful when it gets into contact with or close contact to the superior glenoid with a Neer impingement sign. Another explanation is that the pain is due to contact with other structures not visible arthroscopically. Another factor that could have influenced the results was that the Neer impingement sign could be positive in conditions where the rotator cuff is not visibly torn. Patients with instability and many other diagnoses can have positive impingement signs, and it is possible that in these patients the etiology of the pain was not due to contact of the tendon to the superior glenoid but somewhere else.
An anatomic study performed by Edelson and Teitz80 also supports the contention that a Neer impingement sign does not result in contact between the areas of rotator cuff attachment to the greater tuberosity and the acromion. In their study the simulated motions of the shoulder suggested that the rotator cuff attachment areas made contact with the superior glenoid and that the acromion made bone contact distally on the tuberosity and shaft of the proximal humerus.
In our laboratory we used electromagnetic sensors inserted into the clavicle, scapula, and humerus to measure the relationship of the proximal humerus to the acromion, coracoid, and coracoacromial ligament.86 The cadaver arms were moved from the side into a Neer impingement sign position, and real-time motion was determined using this model. The model calculated the location on the proximal humerus where the shortest distance was to the above structures.
This study found that with a Neer impingement sign, the closest distances of the proximal humerus were between the coracoacromial ligament and a portion of the greater tuberosity distal to the rotator cuff attachments. This study found that the supraspinatus footprint moved past the edge of the acromion in the first 20 degrees of flexion. This study also supports the contention that with forward flexion, shoulder pain may be due to contact of the rotator cuff with structures other than the rotator cuff.
The preceding discussion highlights many of the unknowns in the evaluation and treatment of rotator cuff disease. The concept of impingement is undergoing a metamorphosis as more knowledge is obtained on how the shoulder functions in normal states and in disease. As a result, the authors recommend that the symptoms of rotator cuff tendon inflammation be called rotator cuff disease and not impingement. The ability to make an accurate diagnosis needs to be tempered by the gaps in our knowledge of what causes rotator cuff disease. Uncertainty about what causes the pain in rotator cuff disease not only limits the interpretation of the physical examination but also makes definitive statements about treatment and the interpretation of the results of treatment difficult.
Impingement in Athletes
The study of rotator cuff disease in athletes has begun to change how we think about rotator cuff disease in general. One goal that has proven elusive in treating overhead athletes is the exact etiology of the pain they experience. Although many of the athletes who require surgery for a painful shoulder may have a partial rotator cuff tear and a SLAP lesion, it is not established if these injuries are causally related. It is possible that these pathologies may be due to another entity, such as a lesser form of anterior instability, as theorized by F. Jobe. The role of internal contact in the development of rotator cuff pathology and pain in the throwing athlete remains unknown. As a result, assessment of the athlete with a painful shoulder should include a variety of observations and tests designed to evaluate each of these possible etiologies. These issues are discussed in more detail in Chapters 5 and 6.
Examination for Rotator Cuff Disease (Impingement)
Pain Distribution
Pain is the primary clinical manifestation of rotator cuff disease.10 Localization or distribution of pain is an important point in the differential diagnosis. Pain in the trapezius and rhomboid area is seen commonly with cervical disk disease, fibrositis, and fibromyalgia, whereas pain over the lateral aspect of the deltoid muscle is typically attributed to rotator cuff pathology. The patient, when asked about the pain, typically places his or her hand over the deltoid muscle region (Fig. 4-13). Although radiating pain from the neck to the shoulder is most commonly associated with cervical spine pathology, one must keep in mind that AC joint pain can radiate to the trapezius.
Hawkins and Kennedy24 suggested that in the three stages of impingement lesions, pain was minimal in stage I. They described it as “toothache-like” discomfort that may progress to pain during athletic endeavors. Relief of pain by injecting 10 mL of 1% lidocaine beneath the anterior acromion helped to confirm the diagnosis. In the second stage of impingement, toothache-like discomfort became worse at night, and the patient was unable to perform the requisite maneuver that caused impingement. Pain in stage III impingement lesions may be minimal or severe, toothache-like in nature, often worse at nighttime, and frequently prohibiting any athletic endeavors.
Hawkins and Kennedy further suggested that the “impingement sign” that reproduced pain and the resultant facial expression when the arm was forcibly forward flexed (by jamming the greater tuberosity against the anteroinferior surface of the acromion) was the most reliable physical sign in establishing the diagnosis of impingement. They did not comment on the distribution or pattern of pain produced by this test, however.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree