Chapter 15 Review of the Systemic Effects of Spinal Manipulation
Viscerosomatic effects, polymorphonuclear neutrophils (PMN), natural killer (NK) cells, respiratory burst, heat shock proteins (HSP), tumor necrosis factor (TNF), substance P (SP)
After reading this chapter you should be able to answer the following questions:
| Question 1 | Is there a response in vitro to polymorphonuclear neutrophils challenge after spinal manipulation? |
| Question 2 | Has a change in tumor necrosis factor (TNF) been demonstrated after manipulation? |
Currently it is not possible to determine whether there are identifiable systemic consequences, including immunologic consequences, of vertebral subluxation as defined in this text. The demonstration of such cause and effect relationships requires that stringent criteria are satisfied.1 These criteria include the relative strength of the study designs used to determine causality, the consistency of the association, the temporal sequence of exposure (subluxation) and outcome (systemic effect), and freedom from bias of the diagnosis of a subluxation and the appearance of the presumed outcome (Box 15-1). We have no convincing evidence that a vertebral subluxation causes a systemic effect. What we do know is that spinal manipulation used by chiropractors to treat subluxation elicits some very specific effects on both cells and the concentrations of some soluble factors found in the body that are quantifiable by well-defined techniques. These cells and soluble factors are involved in immune responses, but they play other physiologic roles as well.
BOX 15-1 Criteria to Determine Cause and Effect of the Subluxation
The relative strength of the study designs used to determine causality
The consistency of the association
The temporal sequence of exposure (subluxation) and outcome (systemic effect)
Freedom from bias of the diagnosis of a subluxation and the appearance of the presumed outcome
That spinal manipulation elicits viscerosomatic effects is a concept common to both chiropractic and osteopathy.2–7 Convincing evidence for such effects comes from animal model systems, notably the work of Sato and Swenson,8 who showed that experimental mechanical stimulation of rat spinal cord afferents decreased blood pressure and both adrenal and renal nerve activity. More recently, DeBoer et al.9 demonstrated an inhibition of gastrointestinal myoelectric activity (EMG) in conscious rabbits by experimental manipulation of the thoracic spine, and Deloof et al.10 showed that stimulation of afferents in the central end of the cut vagus nerve inhibited gastric EMG for up to six minutes. In contrast, efforts to demonstrate viscerosomatic effects in humans after spinal manipulation have produced conflicting results. Vernon et al.11 reported a slight but significant increase in β-endorphin levels after spinal manipulation; however, Christian et al.12 were unable to demonstrate differences in the plasma levels of adrenocorticotropic hormone (ACTH), β-endorphin, or cortisol between sham-treated or manipulated subjects before or after treatment. Although it has also been hypothesized that spinal manipulation affects cells of the immune system,4,13,14 until recently little experimental or clinical evidence supported this hypothesis. Vora and Bates’s preliminary report15 that spinal manipulation twice a week for four weeks increased the absolute numbers of B lymphocytes in five of eight patients with documented neuromusculoskeletal disorders has never been repeated.
We have approached the question of systemic responses to spinal manipulation in a number of ways. First, we studied the ability of polymorphonuclear neutrophils (PMN) from both healthy subjects and patients with low back pain to respond in vitro to a particulate challenge after spinal manipulation.16–18 Further, in the healthy patients we explored the plasma concentration of the neuroimmunomodulator substance P (SP), and we investigated the in vitro production of tumor necrosis factor (TNF) by mononuclear cells, primarily lymphocytes.16,17 Second, we applied forces similar to those associated with manipulation to PMN in vitro and measured the production of stress proteins. Stress proteins, also known as heat shock proteins (HSP), are believed to be protective against a variety of stressors. They are highly conserved genetically and are produced by every eukaryotic and prokaryotic organism studied.19 Third, in a small pilot study, we explored the hypothesis that spinal manipulation reduced both pain and plasma levels of prostaglandins, specifically prostaglandin PGF2α in women suffering from primary dysmenorrhea.20 PGF2α is believed to be the putative cause of primary dysmenorrhea. To study cells responsible for the adaptive immune response, we determined the number and function of natural killer (NK) cells and other lymphocyte subpopulations in asymptomatic subjects with a variety of complaints who presented to the National College Chiropractic Center (National University of Health Sciences), our main clinic.21,22 Finally, we examined, as a secondary outcome, lymphocyte subpopulations in patients enrolled in a randomized clinical trial of manipulative therapy for low back pain of mechanical origin.23
Methods
Measurement of Manipulation Forces
The procedures used to measure the forces delivered to the thoracic spine have been described in detail elsewhere.16,24 Briefly, subjects were positioned on a specially constructed force table and were then treated up to six times each with spinal manipulation, using intended force magnitudes ranging from 0% to 100% in increments of 20%. Each manipulation was performed on separate days, with the magnitude delivered on any particular day assigned randomly.
Outcome Measures
In those studies involving the collection of blood, the blood samples were collected by venipuncture in ethylenediaminetetra-acetic acid (EDTA) Vacutainer tubes (Becton Dickinson, Rutherford, N.J.). The blood was collected 15 minutes before treatment and 15 minutes after treatment in the PMN, SP, and TNF studies. In the primary dysmenorrhea study, the postintervention blood sample was collected 60 minutes after treatment because it takes that long for preexisting PGF2α to clear the circulation. Isolation of cells was performed within 30 minutes of collection over a modified Ficoll-Hypaque gradient as previously described.16 Plasma was separated by centrifugation and stored frozen at either −20° C or −70° C until assayed for the analyte of interest. Both SP and PGF2α are stable for at least a year when stored frozen. We analyzed all samples within a month of collection. In the randomized clinical trial, blood was collected at the initial visit, at the twelfth visit, and again after a two-week no-treatment follow-up period. Lymphocyte profiles were determined within 1 hour of collection of the blood sample.
Perceived abdominal and back pain were measured in the women with primary dysmenorrhea with a visual analogue scale, and the effect of menstrual distress on the activities of daily living was measured with the menstrual distress questionnaire.20 The respiratory burst (RB) of PMN was measured using the chemiluminescent (CL) response to an in vitro challenge with a standardized suspension of opsonized zymosan. Endotoxin-stimulated TNF production by cultured mononuclear cells was determined in the culture supernatant solutions by a standard cytotoxicity assay using actinomycin D-treated 1929 mouse fibroblasts.6 Enumeration of lymphocyte subpopulations was performed using cell surface-specific monoclonal antibodies labeled with fluorescent dyes and either conventional fluorescence microscopy or flow cytometry.22,25 Functional assessment of NK cell activity was made with a standard 51Cr release cytotoxicity assay.25
The soluble factors measured in the plasma of subjects in these studies were the neuroimmunomodulator SP and the prostaglandin PGF2α metabolite, 15-keto-13, 14-dihydro-prostaglandin F2α (KDPGF2α). Substance P was determined by radioimmunoassay (RIA) using commercially available reagents after a petroleum ether extraction.16,26 KDPGF2α was determined in the women enrolled in the dysmenorrhea study by RIA as previously described.20
Results and Discussion
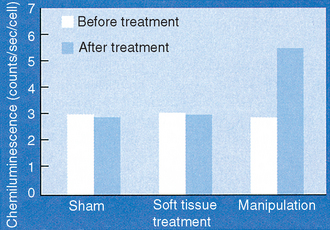
Polymorphonuclear cells isolated from apparently healthy subjects or from patients with diagnosed low back pain of mechanical origin who receive a single spinal manipulation to the thoracic or the lumbar region of the spine are primed to respond to a particulate zymosan challenge with an enhanced RB as measured by CL. Results from a typical series of patients are shown in Figure 15-1. The mean before-versus after-treatment peak counts/second/cell difference in response for cells from subjects receiving a thoracic spine manipulation ranged from 2.2 to 2.9, depending on the study.16–18,27 The magnitude of the after- versus before-treatment enhancement of the RB was similar in PMN isolated from subjects who received a manipulation to the lumbar spine, ranging from 2.15 to 3.2 peak counts/second/cell.25,27 The P values for these data, based on paired students’ t-tests, were consistently less than .001. The force threshold for this response was found to lie somewhere between 450 and 500 N for the thoracic spine.24 Representative manipulation force magnitudes for the manipulative versus the sham procedure are shown in Figure 15-2. The force threshold for this biologic response delivered to the lumbar spine is estimated at approximately 400 N (Triano, unpublished). When we examined the in vitro endotoxin-stimulated production of TNF by mononuclear cells isolated from subjects who had received a thoracic spine manipulation (Table 15-1), we found that there was approximately twice as much endotoxin-stimulated TNF produced by cells isolated after manipulation compared with the production by cells isolated before manipulation.17 Similarly, manipulation of the thoracic spine resulted in approximately a twofold increase in the concentration of plasma SP. (See Table 15-1.) These results strongly suggest that spinal manipulation results in at least short-term priming of PMN for an enhanced RB and also a short-term priming of mononuclear cells for enhanced production of the cytokine TNF. The fact that these priming effects are accompanied by modest but significantly increased plasma levels of the undecapeptide SP suggests that SP is functioning as a regulatory molecule in our subjects as opposed to a mediator of pain. Whether SP or TNF is the proximate priming agent for the enhanced RB of PMN is unclear. However, we suggest that there is a positive feedback loop between TNF, SP, and probably other cytokines as well.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree