Fig. 23.1
Preoperative CT scan for assessment of glenoid bone stock, noting presence of lucency surrounding existing glenoid component
Ideally, the previous operative reports should be obtained to assist in preoperative planning. These reports may provide details of the procedures performed including the status of the rotator cuff at the time of the initial procedure and any specific procedures that were necessary. Most importantly, the previous operative reports should indicate the implants in place. This is critically important for two reasons: first, to assist in obtaining the instruments to facilitate removal; and second, to determine if a platform stem was utilized, thereby potentially allowing conversion to RTSA without stem removal. If previous records indicate a postoperative thromboembolic event, then the postoperative thromboprophylaxis protocol may need to be individualized including preoperative insertion of vena cava filter.
Intraoperative Considerations
Surgical Approach
Patients who have undergone previous arthroplasty will generally have a deltopectoral incision in place or less frequently, a superior incision. Our preference is to utilize a deltopectoral incision, particularly if a deltopectoral incision was previously used, we will utilize the same incision even if its placement may not be ideal. Even if another approach was utilized, we will generally ignore that incision and use a deltopectoral approach because it is the most useful and extensile. It is essential to re-establish tissue planes at all levels beginning with the subcutaneous tissues. The deltopectoral interval should then be developed with mobilization of the subdeltoid space extending into the subacromial space. The pectoralis major may be adherent to the underlying conjoined tendon muscles. Ideally, the plane between these two structures should be developed; however, when scarring is extensive, this can be difficult. In these situations, we concentrate our efforts on mobilizing the conjoined tendon from the underlying subscapularis. This may be difficult especially when the subscapularis is deficient. This dissection should be performed with the humerus in external rotation to minimize proximity to the axillary nerve and the neurovascular bundle. An important landmark is the coracoid process recognizing that dissection lateral to this structure is generally “safe” and any dissection medial to it should be performed with care or if possible, avoided. The question of whether or not to dissect out and visualize the axillary nerve is frequently asked and there is clearly a difference of opinion on this point. Our approach is to avoid this dissection since we feel it is not necessary as long as great care is taken with any dissection about the anteroinferior aspect of the humeral head. We primarily utilize electrocautery for releases in this area as the humerus is externally rotated because we feel it is safer than sharp dissection.
It is essential to obtain adequate mobilization of the proximal humerus by releasing any remaining subscapularis and the inferior capsule such that a full 90° of external rotation can be achieved. Mobilization of the proximal humerus is also achieved by release of the subdeltoid and subacromial spaces. These releases will allow the humerus to be placed in extension and external rotation with tension thereby minimizes the risk of fracture and providing proper exposure of the humeral component. With the initial dissection complete, the humeral side can be addressed.
It is important to emphasize the importance of maintaining a high index of suspicion for infection preoperatively (as described) as well as intraoperatively. We always obtain multiple intraoperative cultures at different areas of the glenohumeral joint, humeral canal (if stem is being revised), and the glenoid. We also have a very low threshold for intraoperative frozen section to assess the presence of polymorphonuclear (PMN) cells per high power field. Although shoulder-specific data on intraoperative frozen section is limited, the data for hip and knee arthroplasty are sufficient for us to consider it an important step if the intraoperative findings are a concern. The topic of infection is considered in detail in Chap. 21.
Humerus
In cases of revision of previous total arthroplasty, humeral loosening is much less common than glenoid loosening [2, 10]. One study found that of 1584 shoulder arthroplasties performed over two decades, 108 were revised, of which 17 required humeral stem removal for loosening. Moreover, survivorship analysis showed that at 10 years, only 8 % of humeral stems required revision, and only 18 % at 20 years [10].
If humeral revision is required, there are three situations that may be encountered: 1. A well-fixed platform humeral stem that may be left in place when the glenoid is removed and replaced with a baseplate and glenosphere for conversion to RTSA; 2. The humeral stem (cemented or noncemented) is grossly loose and thus removed relatively easily—in cases of a cemented stem a cement mantle may remain in place; 3. The humeral stem is a well-fixed non-platform stem (either cemented or noncemented) and removal is required in order to implant a stem that is compatible with a reverse total shoulder system. Each of these possibilities requires careful preoperative planning including knowledge of the implant system and the need for implant-specific equipment in the operating room.
Platform Humeral Stems
Platform humeral stem systems are part of the design features of the following systems (as of the time of this writing): Biomet (Warsaw IN); DePuy (Warsaw IN); Exactech (Gainsville FL); Integra (Plainsboro NJ); and SMR Lima Ltd. (Arlington TX). On occasion, there are those systems whose stems accommodate an adaptor for conversion to reverse, such as the Tornier (Stafford, TX) system and the Plus (Smith and Nephew, Inc.). Knowledge of the specific implant system utilized may avoid the need for removal, of a well-fixed implant.
Shorter operative times and fewer intraoperative complications can be expected when revising a TSA to a RTSA if a platform stem is in place (Fig. 23.2) [11, 12]. Werner et al. [12] found significant improvement in Constant scores and no neurologic complications in a series of 14 patients in which the humeral stems remained in place and conversion to reverse shoulder arthroplasty was performed.
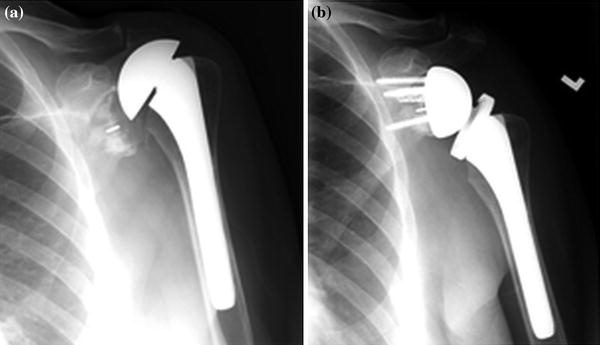

Fig. 23.2
Conversion of total to reverse using platform humeral stem (Exactech)
Loose Humeral Component
The second possibility encountered is a loose humeral component. This can occur in the setting of both a cemented and noncemented stem although it is generally more common with cemented stems. When a loose noncemented humeral stem is removed, we prepare the canal by removing any fibrous tissue, carefully reaming and broaching to a larger size stem with the goal of inserting a noncemented stem. Our preference is to avoid the use of cement if at all possible as long as secure fixation can be achieved. If cemented fixation is necessary, we prefer using cement only proximally and avoided filling the canal. This approach is utilized in consideration of possible future revision which may be necessary particularly if infection develops.
If a loose cemented stem is in place, we proceed with removal of the stem and any loose cement. If there is an intact cement mantle, we prefer to cement a new component into the remaining cement mantle (Fig. 23.3). If a small size stem was in place, then a portion of the cement mantle should be removed using an ultrasound cement removal system in order to allow a stem to be inserted with a 2- to 3-mm cement mantle. If there is radiolucency at the bone–cement interface, then cement should be removed completely to the endosteal bone surface. Once again our preference is to use an ultrasound cement removal instrument to minimize the risk of cortical fracture. Cement removal should include removal of the distal cement and the cement restrictor if this is necessary to implant a new humeral component. When using the ultrasound systems, it is important to maintain copious intramedullary irrigation to minimize the risk of thermal injury to the radial nerve as this has been a reported complication [13].
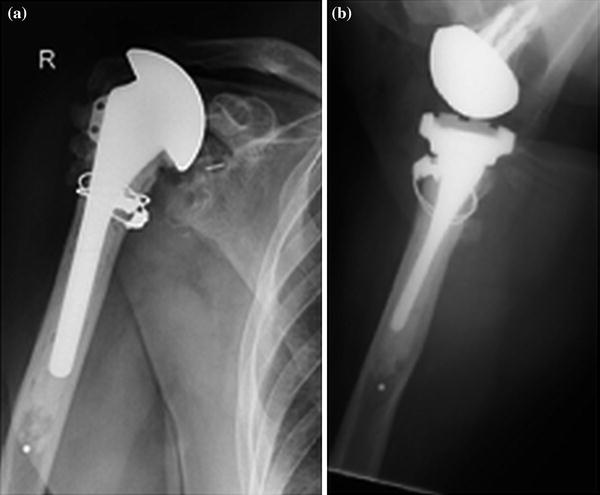

Fig. 23.3
A smaller diameter humeral prosthesis has been cemented into the previous cement mantle
The use of a long humeral stem should be considered if: 1. The component loosening has resulted in significant endosteal erosion creating a potential stress riser at the tip of a standard length stem; or 2. If a cortical breach occurs during cement removal such that a long-stem component is needed to provide intramedullary support.
Well-Fixed Humeral Component
The most difficult scenario encountered is revision of a well-fixed humeral component. The goal in this setting is to remove the component with minimal compromise of bone. For noncemented stems, thin osteotomes should be used to disrupt the proximal bone implant interface. This can be challenging because the geometry of the proximal portion of the stem makes it difficult to pass the osteotomes. For cemented stems, thin osteotomes are used to disrupt the bone–cement interface, with the same challenges as described with noncemented stems. In both cases, it is essential to have the implant-specific instruments for removal. This allows a disimpaction device to be secured to the stem to maximize the chance of removing the component. When this cannot be safely performed, then a humeral osteotomy will be necessary. Different osteotomies have been described in the literature, including the use of a distal anterolateral cortical window, an L-shaped humeral cut, a vertical humeral osteotomy (VHO), and a vascularized humeral osteotomy. All have been used successfully [14, 15].
The distal cortical window approach includes making a window at the distal end of the humeral stem and extending it distally such that access is allowed into the intramedullary canal to dislodge the cement plug and the existing prosthesis. A long-stem component should then be utilized which can bypass the defect by at least 2–3 cortical diameters and augmented with an allograft strut, held in place with cerclage wires (Fig. 23.4).
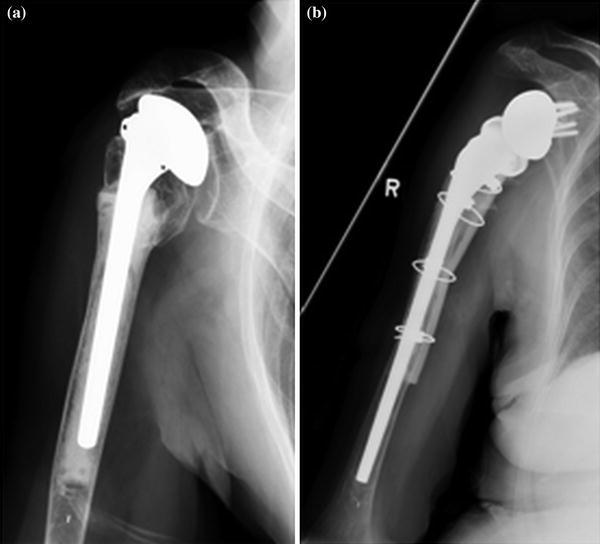

Fig. 23.4
Use of osteotomy for cemented prosthesis removal. Strut allograft and cerclage wires for fixation after placement of new humeral stem
The VHO was proposed to lower the incidence of intraoperative fracture and optimize conservation of bone stock. This osteotomy is described as being made with an oscillating saw lateral to the bicipital groove proximally, and extending approximately 10 cm distally to the interval between the anterior deltoid and pectoralis major insertion. The distal extent is to the insertion of the deltoid, but not distal to the humeral stem. It is then wedged open carefully with the use of multiple osteotomes, thereby disrupting the bone–cement interface. The prosthesis can then be removed with the cement mantle [14]. Using this technique, Van Thiel et al. reported no perioperative fractures in the 23 patients available for retrospective review at an average of 41 months.
We prefer the vascularized osteotomy described by Wright et al. [15]. To briefly summarize this technique, the length of the existing stem and cement mantle from the medial calcar is determined pre- or intraoperatively through radiographs or live fluoroscopy. A 2.5-mm drill is used to perforate the cortex at the lateral margin of the brachialis, forming a vertical trajectory through which an oscillating saw and multiple osteotomes are used to then perforate the cortex. Medial to this, multiple drill holes are placed to form the hinge on the open-door osteotomy and the two vertical limbs are then joined at their distal extent by a horizontal osteotomy performed with an oscillating saw. The key to this technique is preserving vascularity to the proximal humerus by maintaining the majority of the brachialis attachment.
For any of these revision scenarios, even if there are obvious signs of component loosening, it is essential to have appropriate implants and equipment available in the operating room, including cerclage wires, humeral plates, allograft strut grafts, and long-stem components (Fig. 23.5).
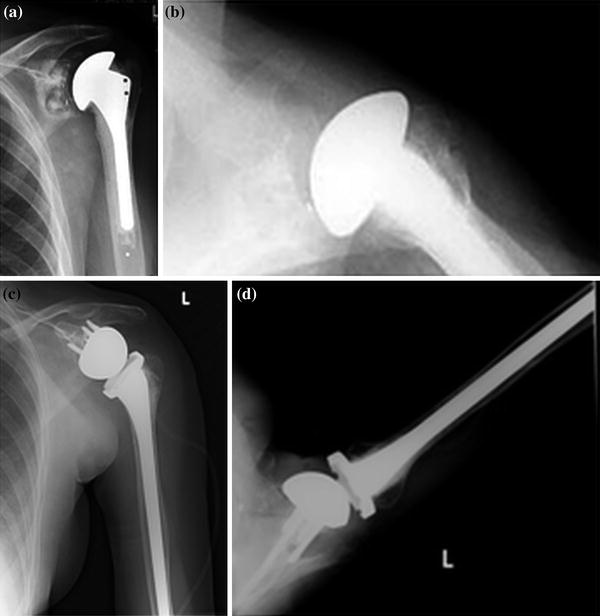

Fig. 23.5
Long-stem humeral prostheses (a). Preoperative radiographs (b–d). Postoperative radiographs
For cases in which significant proximal humeral bone loss is present, bulk proximal humeral allograft has been recommended [16]. This was described as needed to enhance deltoid function and provide bony support for the humeral component. However, a more selective approach has been recommended based upon the amount of humeral bone loss and the amount of medialization of the glenoid resulting from glenoid bone loss [17, 18]. Budge et al. [17] investigated revision of failed TSAs in the setting of proximal humeral bone loss where revision is performed with a reverse shoulder prosthesis and no additional proximal humeral allograft. The authors proposed that the stability conferred by the semi-constrained properties of the reverse implant in addition to its ability to function in the absence of attachments for the rotator cuff muscles would allow it to confer sufficient stability to allow good function even with deficient proximal humeral bone. Indeed, in their published series, the authors found that in cases where a reverse prosthesis is used for the revision of failed total shoulder constructs that have an average proximal humeral bone loss of up to 4 cm, there were good functional outcomes despite the fact that no proximal allograft was used, and there was furthermore, no evidence of humeral loosening during a minimum 2-year follow-up period (refer to Chap. 22 on RSA in the setting of humeral bone loss).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








