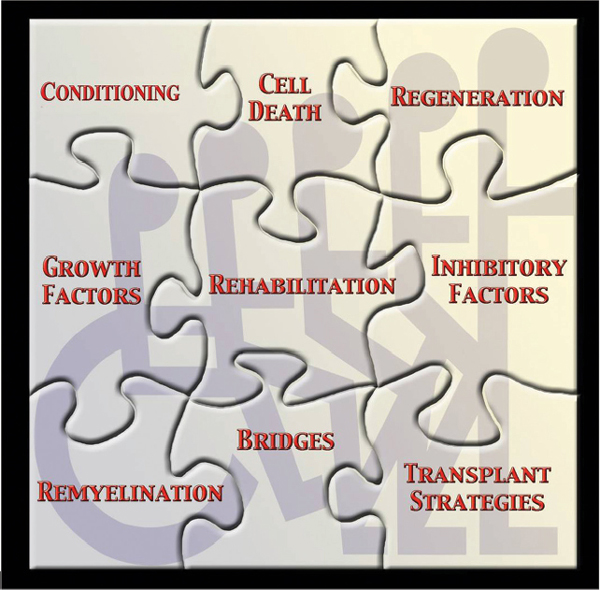
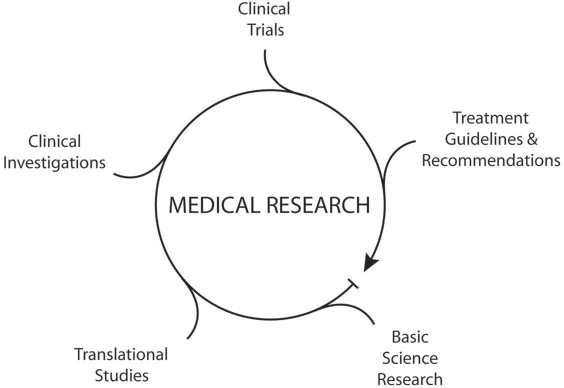
28 Key Points 1. Spinal cord injury is a complex clinical problem. 2. Translational studies provide a solid basis for clinical investigations. 3. A multidisciplinary approach brings together required expertise to target this condition. 4. Core facilities can provide the capability to conduct difficult procedures. 5. Discovery research generates new knowledge on which to base future questions. 6. A bench to bedside approach in translating new discoveries is required to advance this field. Traumatic injuries to the central nervous system represent a complex insult to the most complicated organ in the human body. Injuries to the spinal cord produced by primary insults initiate a series of secondary injury mechanisms that each by itself can participate in the eventual destruction of tissues and long-term neurological disorders. Although significant progress has been made in understanding the pathogenesis of spinal cord injury (SCI) and the identification of novel targets for therapeutic interventions, today there are no treatments that have been shown to be effective when tested in phase 3 clinical trials in SCI patients.1 Although the use of methylprednisolone is recommended following SCI in specific cases, its use has declined over the last several years due to emerging information regarding the harmful side effects of this steroid. Thus, continued investigations into injury mechanisms initiated by SCI as well as the development of novel therapeutic interventions to protect and promote reparative strategies are critical. Because of the complexity of SCI, including a wide range of cellular, molecular, and biochemical responses, it is clear that more work is required to help identify novel treatments to target this patient population in well-designed clinical trials. Because of the complex nature of SCI and the multifactorial nature of its immediate and later-occurring consequences, it is critical that research programs addressing this clinical problem are multidisciplinary and include expertise from different research areas (Fig. 28.1). Such a multidisciplinary approach allows for a wide range of expertise to tackle different aspects of the injury and treatment process.2 To this end, various SCI centers have been established that attract a range of scientists and clinicians interested in making a difference in these patients’ lives. As we move closer to testing our basic science discoveries in translational models of SCI and ultimately to initiate clinical trials (Fig. 28.2), it is clear that there are specific steps that need to be included in the investigative process to make such a translational program successful (Fig. 28.3). This chapter reviews several of the prerequisites that are felt to be critical as we attempt to move new therapies to the clinic. As already mentioned, SCI is a complex clinical problem that is relatively difficult to replicate in the laboratory. Over the past 25 years, various models of SCI have been developed that mimic many of the aspects of human SCI.3 Although no one model exactly mimics all aspects of human SCI, various models do reproduce in a consistent manner many of the biomechanical aspects and cellular response characteristics that occur in an injured patient. These injury models include spinal cord transection, clip compression, and contusive injury. Using other approaches, SCI is produced by occluding feeding blood vessels, producing a focal ischemic insult leading to long-term paralysis. The importance of having multiple models in a translational laboratory is that the heterogeneous nature of SCI can be replicated with the various approaches. Also, it is anticipated that, if a particular therapy works in one type of SCI model, it might be advantageous to test that promising therapy in another model that may mimic another subpopulation of individuals with SCI.4 Fig. 28.1 The puzzle diagram indicates different research disciplines that are required for the successful development of therapies to promote protection and recovery after spinal cord injury and emphasizes major components of a multidisciplinary research program needed to achieve this objective. Fig. 28.2 Various steps for moving a discovery from the bench to the bedside are necessary. It is advantageous for translational research programs to incorporate each of these steps. Another consideration regarding SCI is the level of injury. Recent models have allowed reproducible injuries to be produced at specific levels, including the most common cervical and thoracic levels. Currently, a high frequency of SCI patients sustain cervical SCI.5 Thus, although in the past more conventional thoracic injuries were frequently produced and evaluated for motor assessment, more recent studies have concentrated on cervical injury, leading to reproducible histopathological and behavioral deficits. Importantly, clinically relevant behavioral consequences, including forelimb function and decreased hand function, can be evaluated.6,7 In a successful translational research program in SCI, it is important to consider evaluating new therapies in both cervical and thoracic models. Another variable regarding injury models is whether the model mimics mild, moderate, or severe injury. As previously mentioned, the SCI population includes a range of injury severities that can be replicated in a controlled laboratory environment. In contrast to severe injury, mildly and moderately injured animals show more robust evidence for spontaneous patterns of functional recovery that sometimes can make the assessment of therapeutic interventions challenging. Thus the use of SCI models that take into consideration injury severity and the heterogeneity of human SCI will maximize the chances of finding effective therapies that protect and promote recovery in the clinic. Fig. 28.3 Multidisciplinary research programs targeting SCI may include several key elements. A variety of functional outcome measures are used to assess injury severity and therapeutic interventions in animal models of SCI. The Basso, Beattie, Bresnahan (BBB) Locomotive Assessment Scale is routinely used to evaluate locomotive function in rodents following thoracic SCI.8 This 21-point scale is an advantageous method to assess different aspects of walking over a repetitive testing period. Other motor tests, including the inclined plane, number of foot faults, and beam walking, can provide important information regarding deficits in motor function that are so critical to the human population with SCI. In terms of cervical injury, new testing strategies are now being conducted to evaluate forelimb function, including hand grip and strength. The ability of animals to remove pellets from a cylinder allows investigators to more critically evaluate fine motor hand skills after SCI.9 Over the last several years, abnormal sensation following SCI has become an important quality of life issue in terms of modeling SCI in the laboratory.10 A significant number of patients show abnormal sensations, including neuropathic pain, that seriously affect their ability to carry out normal daily functions. Thus more emphasis is being placed on assessing sensory function through a variety of approaches after SCI. Because some treatment strategies may have the potential to reduce or aggravate neuropathic pain, these types of sensory outcomes are critical in preclinical work. In the majority of SCI preclinical studies, histopathological approaches are used to evaluate contusion size as well as percentages of gray and white matter sparing following various treatment strategies.11 Nonbiased stereological approaches can be recommended to quantitate numbers of cells either by routine histological stains or by immunofluorescent approaches. These types of morphological strategies allow for the characterization of neuropathological changes at the injury site or occurring in spinal cord levels rostral or caudal at specific times after injury. Because different treatments may selectively affect gray or white matter pathology, the assessment of both gray and white matter vulnerability and sparing after treatments is critical as we attempt to move our discoveries forward. Confocal microscopy is also being used to identify endogenous and migrating cell types in spinal cord tissues after injury and to evaluate treatment effects on cell survival and other cellular responses to injury.12 With immunofluorescent approaches, cells can be labeled with one or more specific antibodies that allow critical questions to be asked concerning the phenotype or function of a particular cell. These imaging approaches are becoming very useful to investigate spinal cord regeneration,13 endogenous reparative responses to trauma and cellular transplantation strategies to promote reparative processes and recovery.14 Electron microscopy continues to be an important tool in the area of SCI where evidence for axonal demyelination and remyelination is frequently seen. Ultrathin sections are examined with a transmission electron microscope to clarify the ultrastructural characteristics of various cell types and complement the evaluation procedures using other microscopic approaches that have been discussed. The availability of these different morphological outcome measures provides important information regarding the structural integrity of the tissue as well as the effects of various treatment strategies. In many instances, established core facilities can facilitate the successful steps necessary for an effective translational program. Core facilities that involve animal surgery, for example, can recruit personnel with expertise in producing SCI. Core facilities can maintain expensive instruments, including SCI devices, that therefore do not have to be replicated in multiple principle investigator laboratories. This approach helps maximize the consistency of lesion production and reduces potential drift in injury severity that can be seen over time. The increased use of transgenic mice has emphasized the need for well-maintained transgenic facilities within research programs. These facilities are built especially to house and breed these expensive animals for research programs. Special needs, including environmental filters and care of the animals to enhance breeding, are all important for these types of facilities. An important component of translational programs is critical animal care after SCI. SCI animals are highly susceptible to infection and require special attention to maximize good outcome and long-term survival. These animals can become infected and require care to reduce discomfort and other consequences of the injury. Personnel experienced in caring for these animals, including bladder expression and conducting other steps that ensure the health of the animals, are critical. As discussed, behavioral testing is an extremely important clinical outcome measure that is commonly used in SCI investigations. Several different behavioral tasks are often utilized, and expertise is required to ensure that the behavioral tasks are conducted and scored properly. Testing must be done in a blinded fashion so no information is given to the evaluators regarding the various treatment groups. Thus, randomization and blind assessment strategies are necessary as interventions are assessed. Because behavioral testing apparatuses are expensive, a behavioral core facility allows for the maximization of the equipment and alleviates the need for similar devices to be purchased in multiple principal investigator laboratories. A histopathology/immunocytochemistry core also allows investigators to have tissues processed for routine histopathological approaches, including paraffin, frozen, or vibratome sectioning. This core ensures the quality and consistency of the product in terms of embedding, sectioning, and specimen staining. Automatic processing machines allow for the tissue to be embedded by the core, again with the appropriate expertise to minimize damage to the tissue. Once the tissue is embedded, technicians qualified to cut the tissue allow consistency of cutting and staining and maximizing tissue availability. Imaging cores can contain and maintain expensive microscopes and other equipment that are used by multiple investigators using a sign-up sheet approach. Confocal microscopes, transmission electron microscopes, fluorescent microscopes, and double-head scopes are made available to the investigators in such a core environment. Recently, the critical need for nonbiased stereological approaches for evaluating histopathological outcome has been emphasized in the scientific literature. Thus various approaches, including multiple imaging stations that allow for the use of nonbiased approaches for the evaluation of tissue responses to injury, are best located in these core facilities. One of the exciting areas of SCI research is the use of cell therapies to protect and promote recovery.14 Various cells, including adult human cells, stem cells, or engineered cells, are transplanted to enhance protection and repair after SCI. Thus it is important that core cell culture facilities also be considered in a successful translational program. These cores are set up and run with the appropriate technical staff to maintain healthy cultured cells as well as to prepare cells for transplantation. Other specialty cores, including viral vector or high content screening, are now being added to the list of core programs to emphasize the cutting-edge nature of our research field. One question that scientists commonly debate at traumatic brain injury and SCI conferences is what specific information should be required by the scientific community prior to moving a new treatment into the clinic. It would be helpful if there were a defined road map to follow in testing a particular drug or other agent in preclinical models prior to human testing. Many agree that some type of replication studies would be very advantageous prior to moving new therapies to the clinic.11 Using this approach, published data from peer-reviewed manuscripts that are clinically relevant are considered for replication by independent laboratories. Fortunately, the National Institutes of Health/National Institute of Neurological Disorders and Stroke over the last several years has funded several laboratories to conduct these replication studies for this particular reason.11,15–17 It is felt that, if studies can be successfully replicated and published, it may provide a strong rationale for moving the studied therapy to the clinic. Similar strategies are also being considered in other clinical fields, such as traumatic injury and stroke, to enhance the successful translation of preclinical findings. In these cases, multiple laboratories using different models of central nervous system (CNS) injury will test similar therapeutic strategies to document efficacy. It is felt that if multiple laboratories show efficacy of a particular agent using their own established injury models and outcome measures, it is strong evidence that these treatments may prove efficacious in a heterogeneous patient population. Because of the large number of failures that have been reported in clinical trials targeting CNS injury, it is suggested that established translational research SCI programs be involved in replication studies supported by the scientific community. As previously discussed, the complexity of human SCI demands the involvement of many types of scientists and clinicians who can provide specific expertise as we think about this clinical problem. For example, cell biologists are required to understand the normal function of cells and what surface or intracellular mechanisms might be appropriate for targeting therapies to stop destructive mechanisms.12 Developmental neuroscientists have become very important because of their expertise in brain and spinal cord development, including complex cellular maturation processes and the role of guidance and inhibitory molecules in circuit formation and target recognition during development. These concepts, which were first described in the developmental literature, now are being evaluated and discussed in the acute CNS injury field. Systems neuroscientists bring critical expertise in terms of circuit and synaptic function underlying the behavioral consequences of SCI. Because of the complexity of the spinal cord, including injury severity and potential for circuit dysfunction and plasticity that may occur after injury, it is important that these investigators be included in the investigations. As already emphasized, functional testing involving behavioral and electrophysiological studies is critical as we evaluate therapies and the consequences of injury. Scientists trained in the fields of psychology and animal behavior are critical as we develop clinically relevant outcome measures that may help predict successful clinical trials. Electrophysiological expertise continues to be important as we assess cellular responses to injury and evaluate circuit function and plasticity. A critical component of a translational spinal cord research program is the involvement of clinicians at all steps of the investigative and discovery process. Clinicians who treat SCI patients regularly can bring to the laboratory useful information regarding clinical questions that merit investigation. While moving bench findings to the clinic, it is also clear that clinical problems or questions that require attention be introduced into the preclinical setting for the basic scientists to work on. Therapeutic approaches that are being anticipated for clinical testing need to be discussed with treating physicians to determine their clinical relevance as well as potential risk factors. Because of the emergence of bioengineering in the area of restorative strategies to improve function in the disabled population, the communication between laboratories doing medical research and biomedical engineering should also be enhanced.18,19 This research team approach invites many different types of experts to focus on the problem of SCI and allows for a holistic approach to be concentrated on this complex clinical problem. The regulatory guidelines for moving new discoveries to the clinic include multiple steps and approaches.20 Many of these steps are not routinely used in laboratories where animal models of injury are being produced and novel treatments are tested. Therefore, expertise in US Food and Drug Administration (FDA) regulations and guidelines is critical as one attempts to obtain approval for new treatments. Preclinical studies assessing efficacy, risk factors, toxicity, or other factors that could influence or alter a drug’s effect on a patient have to be fully characterized prior to initiation of a trial. In effective translational research programs, that type of regulatory expertise can be found within the university setting or through consultant agreements that allow a successful stepwise approach to moving things forward for FDA approval. In addition to FDA guidelines, specialized facilities are also required to obtain FDA approval for a new treatment.21 Good laboratory practice (GLP) is required on some aspects of the preclinical studies to ensure quality control of the various experiments. GLP facilities can be partnered with companies that have expertise in these approaches. Good manufacturing practices (GMP) are also critical as one processes cells or drugs for clinical use. Again, expertise is required to ensure that these procedures are done properly with appropriate reagents and paperwork requirements. As one attempts to move discoveries forward, it is clear that various clinical programs are required to make such a program successful. In traumatic SCI, for example, departments of emergency medicine, neurosurgery, clinical care units, neuroradiology, and rehabilitation all are critical components of the patient treatment programs. Thus it is important that these various departments and programs be integrated into the translational research program so that clinical expertise at different levels can be incorporated into the overall treatment strategy. It is clear that an SCI patient goes through various phases of treatment, evaluation, and care, and a smooth transition from one treatment phase to the next is required for optimal benefit of the various treatment modalities. In the acute setting, stabilization of the patient with attention to physiological variables is of critical importance to limiting secondary insults and injury mechanisms.22,23 In the critical care unit, management of the patient for days after injury necessitates expertise in these areas. Surgical strategies, including decompression procedures as well as stabilization of the spinal column, also offer the patient the best opportunity to recover from the injuries. When appropriate, rehabilitation strategies need to be introduced that will maximize recovery patterns and improve the ability of the individual to have a good quality of life.24 In today’s world, the importance of intellectual property and patent applications is becoming ever more important. Research discoveries that have the potential for translation into the clinic require significant amounts of funding to support clinical studies and trials. Thus the ability to obtain intellectual property rights or patents for these particular discoveries will help investigators move their science to the clinic. Alternatively, funding for investigations can be obtained through federal grants and various SCI foundations. The ability to have intellectual property expertise in a university setting to support translational research greatly enhances the ability to move discoveries into the clinic. Another important function of an integrated SCI center is to train the next generation of scientists to conduct spinal cord research. Many center faculty are associated with basic science, clinical departments, and various graduate programs. Thus there is the opportunity to recruit young trainees, including high school, medical, and graduate students and postdoctoral fellows into the laboratory. Also, visiting scientists from universities and hospitals can spend dedicated research time on conducting SCI studies. These training experiences establish lifelong relationships between the scientists and institutions. As new information regarding recent advancements in SCI become available, it is critical that consumers be introduced to existing support groups and have a place to be educated and informed regarding progress. Established research centers and programs can take a lead role in providing SCI awareness and in directing individuals to appropriate surgical and rehabilitation programs. Thus education, training, and the promotion of awareness of SCI programs and discoveries are important functions of translational research programs. Through tours, presentations, and information placed on SCI program Web sites, all of these strategies can be efficiently communicated to the public. Most scientists rely on research funds to support their programs through grant applications to the federal government as well as other SCI funding agencies. Grant applications usually require a significant amount of preliminary data and remain extremely competitive. Another complementary strategy that is used to obtain research funding is through philanthropy and fundraising efforts. Grateful patients as well as individuals interested in neuroscience are introduced to the subject matter and progress being made in the field. Funds available through philanthropy can allow for the purchase of expensive pieces of equipment, provide startup funds for new collaborations, as well as support studies that, although high risk, may have significant implications for the field. Scientists and laboratories thus need to consider multiple sources of funding to support their research programs. An effective translational research program targeting the area of SCI today is multidisciplinary and involves different types of scientists and clinicians. SCI is an extremely complicated problem and therefore requires a concentrated effort from various investigators bringing time and effort into this exciting but difficult field. These are truly exciting times in the area of SCI, with new treatments on the horizon and clinical trials being proposed and conducted. Only with the continued support of the private and research communities will the successful translation of our basic science discoveries be moved into treatment of patients with paralysis. Pearls
Research in Spinal Cord Injury: Building an Effective Translational Research Program
 Spinal Cord Models
Spinal Cord Models

 Functional Outcome Measures
Functional Outcome Measures
 Structural Outcome Measures
Structural Outcome Measures
 Core Facilities
Core Facilities
 Replication Studies
Replication Studies
 Research Team Approach
Research Team Approach
 Federal Regulations Involving Clinical Trials
Federal Regulations Involving Clinical Trials
 Translational Clinical Programs in Spinal Cord Injury
Translational Clinical Programs in Spinal Cord Injury
 Technology Transfer
Technology Transfer
 Education, Training, and Awareness Programs Neuroprotective and Neuroregenerative Approaches
Education, Training, and Awareness Programs Neuroprotective and Neuroregenerative Approaches
 Fundraising and Philanthropy
Fundraising and Philanthropy
 Conclusion
Conclusion
 Published studies are being replicated by independent groups.
Published studies are being replicated by independent groups.
 These are exciting times in SCI research.
These are exciting times in SCI research.
 Some treatments are being translated to the clinic.
Some treatments are being translated to the clinic.
 Public awareness regarding SCI has been emphasized.
Public awareness regarding SCI has been emphasized.
 Large SCI research groups are working together.
Large SCI research groups are working together.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
