Chapter 38 Reproductive and Hormonal Issues in Women with Autoimmune Diseases
Hormones and Reproductive Immunology
Gonadal Hormones and the Immune System
Gonadal hormones clearly play a role in immune homeostasis. Specific hormones, including estrogen, progesterone, and prolactin, exhibit direct effects on numerous immune cells, cytokines, and apoptosis; and clinical experience suggests that changes in gonadal hormones may modulate disease activity.1 During young adulthood (i.e., childbearing years) the female-to-male ratio for lupus is 9 : 1; however, this female preponderance is not so striking in children and in older adults.2 Furthermore, men with Klinefelter syndrome (47,XXY) appear to have an increased prevalence of SLE, suggesting a role of the X chromosome in the pathogenesis of SLE.3 Different studies have suggested that increased levels of female sex hormones—use of exogenous estrogens as oral contraceptives or postmenopausal hormone therapy and pregnancy—in women with SLE may exacerbate disease.
Female Hormones and Inflammatory Mediators
The regulation of the menstrual cycle or invasion and implantation of the uterine wall by an embryo requires an ebb and flow of inflammatory mediators that are unique to the adult female environment. The need to protect the semiallograft fetus from immune attack without initiating rejection or graft-versus-host disease, while still maintaining an effective immune surveillance and response to infection, indeed sets a high bar for carefully regulated immune response throughout implantation, pregnancy, and parturition. Thus it makes sense that many systemic inflammatory and immunologic responses are mediated, at least in part by female sex steroids—predominantly estrogen, progesterone, and prolactin. For example, progesterone is important in suppressing the inflammatory reaction that would be expected in response to the presence of a foreign body, in this case an embryo. Sex hormones play an influential role on systemic cytokine production and release, largely mediated through the nuclear factor–kappa B (NF-κB). In general, estrogens stimulate a T-helper (Th) cell 2 response and activate antibody production. The production of interleukin (IL)-1, IL-4, IL-6, and IL-10 in macrophages is stimulated by estrogen, as is the production of IL-4, IL-5, IL-6, and IL-10 by Th 2 cells. In contrast, androgens stimulate a Th cell 1 response with the production of IL-1 and IL-12 and activate cluster of differentiation 8 (CD8+) T cells.1 Progesterone may also suppress IL-8 and cyclooxygenase-2 expression, suggesting that progesterone withdrawal at the time of menstruation might promote these inflammatory mediators in preparation for the increased tissue inflammation that accompanies the extrusion process.4
Complex Effects of Sex Hormones on Inflammation
Estrogen replacement in postmenopausal women may increase C-reactive protein5,6 while decreasing a number of other inflammatory mediators.6,7 In the context of the rapidly evolving literature, hormones produced during the ovulatory cycle may normally regulate the complex network of endometrial cytokines.8 However, signal cascades may have counterregulatory effects such that inflammatory mediators that will impact the levels of circulating hormones. For example, lipopolysaccharide, a potent stimulator of monocytes, induces a lengthening of the follicular phase and is associated with decreased estradiol concentrations and increased pituitary release of the luteinizing hormone (LH) and the follicle-stimulating hormone (FSH).9 Complex relationships among female sex hormones, inflammatory mediators, and systemic vasculature are intrinsic to the development of sexual maturity, to the maintenance of the ovulatory cycle in preparation for implantation of the conceptus, and to the maintenance of a healthy pregnancy leading to successful reproduction. However, it is precisely this complicated interplay of female sex hormones and the immune system that sets the stage for the female preponderance of autoimmune diseases including SLE.
Sex Hormones and the Immune and Vascular Systems
Estrogen receptors are found on human monocytes, B cells, and T cells, indicating a direct role for estrogens in the regulation of immune cell activation.10 Overall, estrogen appears to enhance B-cell activation while, at the same time, suppressing T-cell reactivity. Importantly, different circulating levels of estradiol may have differing effects on the immune system; for example, low doses of 17β-estradiol have been found to inhibit IL-6 secretion by human endothelial cells.11 Progesterone is also known as the hormone of pregnancy, because of its profound influence on the cellular, immunologic, and tissue-remodeling changes that are necessary during pregnancy.12 Progesterone induces and hones uterine natural killer (uNK) cells, a variant of NK cells with low spontaneous cytotoxic activity that function locally to induce tolerance to self or fetal antigens. Additionally, progesterone acts to differentiate T-cells into Th2-dominanat cells and the production of IL-3, IL-4, and IL-10. Many of these actions are mediated by a progesterone-induced blocking factor (PIBF).
Inflammation and inflammation-induced coagulation mechanisms are sometimes predictors of future cardiovascular events.13 Because of this, sex steroids may have indirect effects on the risk of vascular thrombosis through immune-modulating effects. For example, estrogen can improve markers of fibrinolysis and vascular inflammation in the arteries of postmenopausal women,7 as well as having other antiinflammatory properties that may have beneficial effects on cardiovascular risk.14 At the same time, estrogen has been shown to increase C-reactive protein,7 a marker of subclinical inflammation independently associated with the increased risk of cardiovascular disease. Many inflammatory cytokines induce adhesion molecules in blood vessel walls, augmenting inflammatory cell adhesion, which may lead to the development of atherosclerosis. One study observed a statistically significant increase in several such adhesion molecules; men and untreated postmenopausal women with coronary artery disease were compared with postmenopausal women with coronary artery disease who were receiving estrogen therapy.15 Clearly, the role of estrogen is multifactorial, with some effects promoting or inhibiting systemic inflammation, as well as the expression of endothelial adhesion molecules of vessel walls. Reasons for this discrepancy may include dose-dependent effects, multiple signaling pathways, and local hormonal and cytokine milieux.
Increased knowledge of the roles of sex steroids in the immune system raises concerns and questions regarding the safety of surges of exogenous or endogenous (during pregnancy) female sex hormones in terms of the diseases of the immune system including SLE. Past observational studies have suggested increased rates of SLE flares with the use of estrogen-containing oral contraceptives or postmenopausal hormone therapy, as well as the use of sex steroids during pregnancy or ovulation induction.16 However, pregnancy and childrearing are an important part of a full and complete life for many women with SLE, and effective contraception is essential for women with SLE to be able to plan pregnancies during times of relative disease quiescence and to avoid fetal exposure to potentially teratogenic medications. Improved understanding concerning how underlying autoimmune disease may affect a women’s reproductive health and how changes in female sex hormones over the reproductive life affects underlying SLE are critical to caring for and counseling women with chronic autoimmune disorders.
Maternal-Fetal Immunology
The normal relationship between mother and fetus promotes growth and maturation in contrast to an allogeneic model of destruction of foreign antigens.17 To enable the fetal semiallograft to survive and grow during 40 weeks of exposure to the maternal immune system, that system must undergo a complex modulation of its innate and humoral components, much of which is not well understood. Pregnancy has long been understood as a Th2-predominant condition, during which a shift of Th cells toward a Th2-dominant state, possibly induced by increasing levels of progesterone, is necessary to establish and maintain a normal pregnancy. This theory is consistent with earlier observations that SLE (a Th2-predominant disease) may be exacerbated by pregnancy, whereas Th1-mediated autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis, psoriasis) appear to be characterized by clinical improvement during pregnancy.17 More recently, however, it is becoming increasingly clear that many more components of both the innate and adaptive immune systems are involved in normal pregnancy.18 Furthermore, many of the immunologic changes during pregnancy may be preferentially located at the maternal-fetal interface and may not be accurately sampled using peripheral blood. Before conception, endometrial stromal cells transform into decidual cells that contain T-cell subtypes with immunosuppressive activity. One Th cell subset secretes cytokines that are beneficial or neutral to the fetus, whereas another is thought to prevent colonization with microbial pathogens.18 uNK cells (also known as decidual NK cells) reside in the endometrium in the nonpregnant state but grow in numbers during the late secretory phase of the menstrual cycle and early pregnancy and make up the most abundant proportion of decidual leukocytes.19 These cells appear to lack the level of cytotoxicity toward trophoblasts that is seen in peripheral NK cells toward infected or malignant cells and serve as a source of local inflammatory and regulatory cytokines and angiogenic growth factors that regulate trophoblast invasion.19
The trophoblast and placenta, once considered passive mediators of maternal-fetal immune trafficking, have been increasingly recognized as playing active roles in mediating inflammation while simultaneously maintaining effective host defense.20,21 Villous cytotrophoblasts and syncytiotrophoblasts escape immune-mediated destruction because both express nonclassical major histocompatibility complex (MHC) antigens that prevent trophoblast destruction through the inhibition of lysis by activated NK cells, as well as limit leukocyte cytotoxic activity, suppress proinflammatory cytokine production, and induce T-cell death. Nonclassical MHC antigens also promote trophoblast proliferation and invasion. Altered expression of nonclassical MHC antigens has been linked to recurrent pregnancy loss (RPL) and preeclampsia. Placental expression of Fas ligand (FasL) may also play a role in pregnancy success through the selective deletion of antifetal T-cell clones. In animal studies, binding to the FasL causes death and removal of autoreactive T cells.
Embryologic Development of the Immune System
Although not a completely protected barrier, the presence of an intact trophoblastic cellular barrier prevents the movement of large numbers of immunocompetent cells into or out of the fetus during pregnancy. In contrast, maternal immunoglobulin G (IgG), by virtue of fragment-specific (Fc) receptors in the placenta, is specifically selected for transplacental transfer. Fetal concentrations of IgG subclass 1 exceed those of other IgG subclasses at all time points. Very little IgG is seen in fetal circulation during the first trimester of pregnancy. Levels slowly rise during the second trimester and reach maternal serum concentrations by approximately 26 weeks’ gestation. Maximum IgG transfer across the maternal-fetal interface occurs during the last 4 weeks of gestation, and fetal concentration often exceeds maternal concentration at term delivery.22 Adequate humoral immunity in the neonatal period depends on the circulating immunoglobulins that have crossed the placenta, and fetal blood levels of IgG reflect maternal levels and specificities. Of course, the placenta is unable to differentiate between helpful and pathologic IgG antibodies; consequently, potentially harmful maternal autoantibodies (including anti–Sjögren syndrome antigen A [anti-SSA/Ro], anti–Sjögren syndrome antigen B [anti-SSB/La], and anticardiolipin antibodies [aCL] will pass into fetal circulation and have the potential to exert pathologic effects on the fetus. Additionally, maternal exposure to IgG-based pharmaceutical agents will lead to passage to fetal circulation.
Reproductive Issues in Women with Systemic Lupus Erythematosus and Related Autoimmune Disorders
Contraception
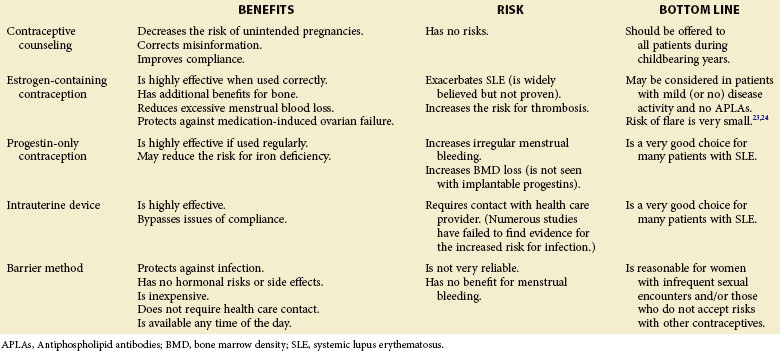
Effective and safe contraception is important for women of child-bearing age to be able to avoid unplanned pregnancies; this control is even more critical for women with underlying SLE because of the additional need to plan pregnancies around periods of relative disease quiescence and to avoid antenatal exposure to potentially teratogenic medications commonly used for the treatment of disease, including mycophenolate mofetil, warfarin, methotrexate, and angiotensin-converting enzyme (ACE) inhibitors. In the absence of cyclophosphamide-induced premature ovarian failure (POF), women with lupus have normal fertility and are at risk for unintended pregnancy without the use of effective contraception. Because of concerns about exacerbating the disease with the use of exogenous estrogens, estrogen-containing contraceptives have been considered relatively contraindicated in women with lupus until fairly recently.16 Two large multicenter trials have recently demonstrated that estrogen-containing oral contraceptives may be used with low risk of disease flares in women with quiescent to -mild disease activity and without antiphospholipid antibodies (APLAs).23,24
Irrespective of the decision to use or to avoid using estrogen-containing contraceptives, many other safe and effective options are available for women with SLE; however, patients continue to be at increased risk for unintended pregnancy because of the inconsistent use of contraceptive methods or the use of contraceptive methods that are unreliable. Even in the general population, approximately 50% of pregnancies are unintended,25 and women taking potentially teratogenic medications do not necessarily use effective contraception more consistently than women without medication exposure. A study of nearly 500,000 reproductive-aged women in northern California found that 77,378 were prescribed a potentially teratogenic medication (U.S. Food and Drug Administration [FDA] pregnancy category D or X) over a single year. Of these women, approximately 50% had no contraceptive method dispensed (e.g., prescription of hormonal contraception, insertion of intrauterine device [IUD], surgical sterilization), and fewer than 50% had any documentation of contraceptive counseling.26 Fortunately, women prescribed category D or X medications were less likely to become pregnant than women prescribed category A or B medications (1.0% versus 1.4% prescriptions). Among a cohort of 222 women with SLE of reproductive age, 42% were at potential risk for becoming pregnant (they were sexually active, premenopausal, and not surgically sterile).27 Of these, 59% reported no contraceptive counseling in the past year. The majority of women in this study reported consistent use of contraception; however, most relied on barrier methods rather than the more effective hormonal contraceptives or IUDs. These results were not changed when the cohort was limited to women taking potentially teratogenic medications.27 Another questionnaire-based study of women with SLE found that 46% of women attending a lupus clinic in the United States were at risk of becoming pregnant; of these, 23% reported routine unprotected sex, and 55% reported at least one occasion of unprotected sex.28 The minority of women (35%) were using hormonal contraceptives or an IUD. Taken together, it is clear that women with SLE require contraceptive counseling concerning the risks of unintended pregnancy, as well as the risks and benefits of different contraceptive options. A summary of contraceptive options is presented in Table 38-1.
Estrogen-Containing Contraception
The use of estrogen-containing contraceptive methods has, in the past, been very controversial. Benefits of these methods include positive effects on bone density, contraceptive efficacy, reduction of excessive menstrual blood loss, and protection against cyclophosphamide-induced ovarian failure; however, these benefits need to be considered in the context of possible risks of increased thrombotic potential and the theoretical risks of exacerbating disease activity.16 Two recent randomized clinical trials have been published to help define the safety and efficacy of estrogen-containing combined oral contraceptives. The Safety of Estrogens in Lupus Erythematosus–National Assessment (SELENA) trial, conducted in the United States, randomized 183 women with SLE of childbearing potential to oral combined contraceptives or placebo for 12 months. To be eligible, women needed to have clinically quiescent or mild, stable disease (i.e., Systemic Lupus Erythematosus Disease Activity Index [SLEDAI] < 4) and agree to use barrier methods of contraception throughout the study.23 Important exclusion criteria included moderate to high titer anticardiolipin (aCL) antibodies, lupus anticoagulant (LA), or any history of thromboses. In this noninferiority study, the primary endpoint of the study was severe SLE flares. Results showed that in this group of women with mild disease, the rates of severe flares over 12 months were not different among those on estrogen-containing contraception and placebo (7.7% versus 7.6%); in addition, the rates of mild to moderate flares were no different between the two groups (1.40 versus 1.44 flares per person per year). One case of deep-venous thrombosis occurred in each group, as did low numbers of pregnancies.23 Similarly, a single-center randomized study of three contraceptive methods was performed in Mexico. In this study, 162 women with SLE were randomized to either estrogen-containing combined contraceptives, progestin-only pill, or a copper IUD for 12 months.24 This study was also designed to compare disease activity among the three groups; exclusion criteria included active disease (SLEDAI score higher than 30) and any history of thrombosis. Rates of SLE flares were similar among the three groups (incidence density rates of 0.86, 1.14, and 0.91 for combined oral contraceptives, progestin-only pill, and IUD, respectively). The incidence of severe flares was similar among groups—two, four, and two flares in estrogen, progestin, and IUD groups, respectively). Two deep-venous thromboses occurred in each of the hormone-containing arms (all of whom had low positive APLAs), and one to two pregnancies occurred in each group.24 These data provide convincing evidence that overall and severe flare rates do not appear to be significantly increased in women using estrogen-containing oral contraceptives. Guidelines for the use of oral contraceptives in women with SLE, based on these studies, are listed in Box 38-1. The important caveat is that women with active disease, renal disease, and antiphospholipid antibody syndrome (APS) were excluded from the study—arguably the very patients who are in the greatest need of pregnancy prevention.
Box 38-1
Guidelines for the Use of Oral Contraceptives in Women with Systemic Lupus Erythematosus
1. Inactive or stable or moderate disease activity
2. No history of venous or arterial thrombosis
3. IgG APLAs < 40; IgM APLAs < 40; IgA APLAs < 50; no circulating lupus anticoagulant (unknown if presence of low to moderate titer of APLAs in the absence of a previous thrombosis is contraindicated)
6. Lowest dose of ethinylestradiol (30-35 µg) for combined pill
7. Patient without migraine headaches
8. Addition of low-dose aspirin therapy to hormone regimen if risk factors are a concern
Progestin-Only Hormonal Contraception
In women who may not be considered safe for the use of estrogen-containing contraceptives, hormonal contraception using progestin-only compounds remains another option for effective pregnancy prevention.29 Progestin-only contraceptives come in a variety of delivery methods including oral, every-3-month intramuscular injections, a 3-year subcutaneous implantable device, and a levoprogesterone-containing IUD. No data are available that suggest an increase in disease activity with the use of any progestin-only hormonal contraceptive device, including in the Sanchez-Guerrero24 randomized trial of hormonal or implantable contraceptives. Additionally, progestin-only contraceptives do not confer an increased risk for thromboses; therefore they may be safer alternatives than estrogen-containing compounds among women with APS or women with a history of cardiovascular disease. All forms of progestin-only contraceptives are considered highly effective if used regularly. Implantable progestin contraceptives have the additional benefit of 3 years of efficacy that avoids problems of compliance with pills or with quarterly injections performed in office settings. Because progestins work by thickening cervical mucus and thinning the endometrial lining, continued use may lead to reduced menstrual bleeding or amenorrhea in some patients,30 thus potentially reducing iron deficiency anemia in susceptible women.
Potential adverse effects of progestin-only contraceptives include irregular menstrual bleeding that lasts beyond the initial few months of use. Additionally, some women experience weight gain that may lead to the discontinuation of use; large-scale studies have been inconsistent as to whether there is, indeed, a causal relationship.30 An additional potential concern of relative importance in the SLE population is the risk of BMD loss during the use of progestin-only pills or injections as a result of reduced levels of serum estradiol. Fortunately, BMD loss appears to be reversible on the discontinuation of use in the general population, although it has not been studied in patients with SLE. Implantable progestins do not carry the risk of BMD loss because endogenous estrogen levels return to baseline after initial decrease.30
Intrauterine Devices
IUDs are inserted into the uterus, generally by a gynecologist, with contraceptive effects lasting 5 to 10 years. Worldwide, IUDs are the most widely used reversible method of contraception.28 Two types of IUDs are currently available in the United States: copper-containing devices and a hormone-containing device that releases progesterone. Copper IUDs (ParaGuard) remain effective for up to 10 years; because these devices do not contain hormones, they do not cause changes in menstrual bleeding or other symptoms of premenstrual syndrome. Perhaps the more commonly used type is the progesterone-releasing IUD (Merena). This device is effective for up to 5 years.29 In response to the local release of progesterone, the endometrial lining thins dramatically, leading to a reduction or a loss of menstrual bleeding, which again may provide additional benefits to the patient with SLE by preventing menses-related blood loss. Currently available IUDs are excellent options for women with SLE who need effective, long-term contraception but may be reluctant to add another pill to an already complicated medical regimen. IUDs offer the benefit of completely bypassing issues of compliance and require an active process (i.e., scheduled visit to gynecologist) to remove the device and to restore fertility. As with hormone-releasing contraception, fertility is restored shortly after discontinuation, and devices can be used in both nulliparous and parous women.28 These devices do not carry potential risks of BMD loss or thromboses as observed in other hormone-containing contraceptives.
Because of concerns of increased risk of infections and pelvic inflammatory disease with the use of IUDs in decades past, misperceptions persist of the safety of IUDs in immunosuppressed women.28 Numerous studies have since been performed in several populations at high risk for sexually transmitted diseases without evidence of increased infections, compared with women not using IUDs. These studies include women with human immunodeficiency virus,31 women with a history of sexually transmitted diseases, and women with multiple sexual partners.28
Barrier Methods
Barrier methods of contraception, including condoms and diaphragms with or without spermicide, are among the least effective forms of contraception. They rely on consistent and proper use at every occurrence of sexual intercourse and are fraught with problems of compliance. Additionally, they do not offer any benefits regarding the reduction in menstrual bleeding but avoid all risks associated with the administration of exogenous hormones or device failure. However, barrier methods offer a few distinct advantages. They are among the only contraceptive methods that provide protection against infection with sexually transmitted diseases, a particular concern in women who are likely to be taking long-term immunosuppressive therapies and therefore may be at increased risk of contracting infectious diseases. Additionally, condoms and spermicide are inexpensive, do not require a physician office visit or prescription, and are widely available at any time of day. Thus they may be reasonable options for women with infrequent sexual encounters who do not wish to accept the risks associated with hormonal or implantable contraception. Regular use of spermicide with barrier methods will increase the efficacy of barrier forms of contraception.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree