Chapter 12 Rehabilitation and Prosthetic Restoration in Upper Limb Amputation
Limb loss and limb deficiency occur in significant numbers worldwide. Amputations are performed to remove limbs that are no longer functional because of injury or disease. The more common causes are related to diabetes, peripheral vascular disease, trauma, and malignancy. Genetic variation and mutation are the typical causes for congenital deficiencies. Upper limb loss is more commonly due to trauma than lower limb loss. Before 1900, war-related injury was the major reason for limb loss in the United States. Surgical amputation has evolved significantly since the days of severing a limb from an unanesthetized patient and dipping the residual limb in boiling oil to achieve hemostasis. As America became industrialized, there was a rise in civilian trauma causing upper limb loss as a result of crush injury, laceration, and avulsion. We owe a great deal to our wounded military and manual laborers, then and now, for pushing us to develop the technologies and options for those with limb loss today. The evolution of the upper limb prosthesis is founded on the principles of Salisbury and Newton.33 Prosthetic scientists stood on the shoulders of these giants while building functional tools that assist with performing daily tasks. As technologies advance, we are even more dependent on the training and the technical skill of the upper limb prosthetist. Even though we have entered the bionic age, the cable-and-hook systems remain the staple of upper limb prostheses because of their relative versatility and simplicity.
Demographics, Incidence, and Prevalence
In the United States an estimated 185,000 persons undergo an amputation of the upper or lower limb each year.31 In 2008, it was estimated that 1.9 million persons were living with limb loss in the United States (Johns Hopkins Bloomberg School of Public Health, unpublished data). Of this estimate, 500,000 persons were living with minor (fingers, hands) upper limb loss, and 41,000 persons were living with major upper limb amputations.8 Because of the aging of the population and higher rates of dysvascular disease related to diabetes and obesity, it is projected that the number of people living with lower limb loss in the United States will double by the year 2050.8
Trauma accounts for 90% of all upper limb amputations. During the next 50 years, the incidence of amputations secondary to trauma is estimated to remain flat if not decrease.32 The incidence of upper limb trauma is hypothesized to decrease because of more successful occupational safety standards.17 The future is also likely to bring even more aggressive and successful limb reconstruction and reimplantation. Other causes of upper limb loss include burns, peripheral vascular disease, neurologic disorders, infections, malignancies, contracture, and congenital deformities.3
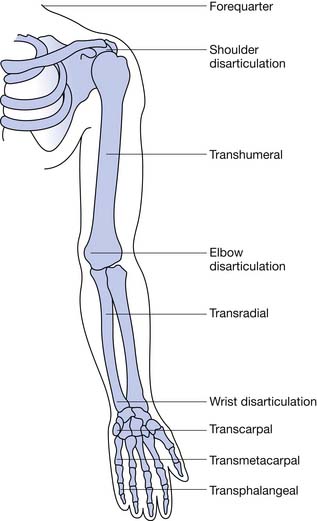
Finger amputation represents the highest percentage (78%) of upper limb amputations reported on hospital discharges.16 Most amputations involve single digits, with the index, ring, and long fingers accounting for 75% and the thumb 16%.3 Excluding finger amputation, the most common upper limb amputations are through the forearm (transradial) and humerus (transhumeral), respectively (Table 12-1, Figure 12-1).42 Most civilian limb injuries that result in amputation occur at work and involve saws or blades (e.g., lawnmowers and snowblowers). Blast-related injuries are rare in the civilian population (8.5%). In the active military, however, amputee injuries are from mortars, gunfire, improvised explosive devices, and rocket-propelled grenades. Because of the extreme forces involved, concomitant injuries such as traumatic brain injury, visual and hearing impairment, soft tissue loss, and burns are common. A fifth of all combat-related major amputations involve the upper limb.14,52 Two thirds of amputations resulting from trauma occur among adolescents and adult younger than 45 years.8 Males account for greater than 75% of those with upper limb loss, and the more severe the injury, the more likely the victim is male.3
Table 12-1 Upper Limb Amputations by Site: 1993-2006
| Procedure | Percentage of Total Upper Limb Amputation Procedures Performed |
|---|---|
| Amputation through the hand | 15 |
| Disarticulation through the wrist | 10 |
| Amputation through the forearm (transradial) | 31 |
| Disarticulation of the elbow | 7 |
| Amputation through the humerus (transhumeral) | 28 |
| Shoulder disarticulation | 7 |
| Forequarter amputation | 2 |
An estimated 4.1 per 10,000 babies are born each year with all or part of a limb missing, ranging from a missing part of a finger to the absence of both arms and both legs. Congenital deficiencies in the upper limb are more common (58%), and they occur slightly more often in boys. The most common congenital amputation is at the left short transradial level. Most cases of congenital upper limb deficiency have no hereditary implications. Congenital limb deficiencies occur because of the failure of part or all of a limb bud to form. The first trimester is the critical time for limb formation, with the bud occurring at 26 days’ gestation and differentiation through the eighth week of gestation. The etiology often is unclear, but teratogenic agents (e.g., medications and radiation exposure) and amniotic band syndrome are two common causes. Maternal ultrasound examination often identifies the limb deficiency before delivery. There have been many descriptions of congenital limb deficiencies (Box 12-1), with the development of the current and preferred system by the International Society for Prosthetic and Orthotics (ISPO; Box 12-2). The ISPO terminology divides the limb amputations into transverse or longitudinal. By definition, a child who has a transverse deficiency has no distal remaining parts. For example, a child with a transverse radial deficiency has a normal upper arm and a portion of the radius, but is missing the hand and fingers. Longitudinal deficiencies have distal portions present with a partial or total absence of a specific bone. The most common congenital limb deficiency in the upper limb is a longitudinal partial or complete lack of the radius. Longitudinal hand reductions represent half of all congenital upper limb reductions, and multiple limb reductions are found in less than 20% of live births.19,46
BOX 12-2 ISPO Classification System for Congenital Upper Limb Reductions
ISPO, International Society for Prosthetic and Orthotics.
Nomenclature and Functional Levels of Amputations
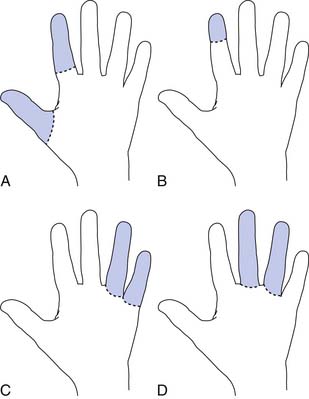
Radial amputations (Figure 12-2, A) involve the thumb and index finger and compromise grasp. Fingertip amputation (Figure 12-2, B) is the most common type of amputation. The thumb is the most functionally critical digit. Thumb amputation, partial or complete, results in loss of palmer grip, side-to-side pinch, and tip-to-tip pinch. Amputation of one of the other digits causes lesser functional loss. Transverse digit amputations occur at one or more digits and can be fit with functional finger prostheses. Ulnar amputations (Figure 12-2, C) involve digits IV and V, and hook grasp is lost. The loss of digit V is functionally under estimated because of this powerful grasp. Central amputation (Figure 12-2, D) involves digits III and IV, and reconstruction is usually not attempted, and a cosmetic substitute is used. The residual limb refers to the remaining part of the amputated limb. The sound limb refers to the nonamputated limb. Wrist disarticulations are rare, but are preferred over more proximal amputations because maximal pronation and supination are preserved.12
Proximal to the hand, amputations are divided into the following categories: transradial, transhumeral, shoulder disarticulation, and forequarter amputation. Depending on the percentage of the limb remaining compared with the sound side, further categorizations can be made such as “short” and “long” to define the residual limb. These categorizations have functional implications. For the transradial residual limb, the longer the length, the more pronation (normal, 120 degrees) and supination (normal, 180 degrees) is preserved. Of the pronation and supination preserved, 50% can be transmitted to the prosthesis.12
Transradial amputations are based on measurements made from the longest residual bone (ulna or radius) to the medial epicondyle. This is then compared with the measurement of the sound side from the ulnar styloid to the medial epicondyle. The remaining length impacts the ability to pronate and supinate the forearm with the prosthetic device. A long transradial amputation preserves 55% to 90% length, allows up to 60 degrees of supination and pronation with a prosthesis, and maintains strong elbow flexion.47 A medium transradial amputation preserves 35% to 55% length, and pronation and supination with a prosthesis are lost. Elbow flexion is reduced because of the inhibiting prosthesis. A short transradial amputation is defined as 0% to 35% preservation, which results in difficult prosthetic suspension and the additional loss of full range of motion (ROM) at the elbow.
The elbow disarticulation creates functional and prosthetic fit difficulties related to suspension and elbow joint placement. This level of amputation preserves humeral rotation to the prosthesis and can be accommodated by modern socket fabrication techniques and cosmesis. It is most suitable for the growing child to preserve the epiphysis for growth.38 The elbow disarticulation is recommended instead of bilateral transhumeral because of functional prosthetic control.
The transhumeral amputation can also be classified into three levels. The more humeral length preserved, the more optimal the prosthetic restoration. The long transhumeral is defined as preservation of 50% to 90% of length relative to the sound side. Glenohumeral motions are preserved and uninhibited by the prosthetic socket. The short transhumeral is defined as preservation of 30% to 50% of length, which results in loss of glenohumeral motion because of the inhibition of the prosthetic socket that encompasses the acromion.47 The glenohumeral motions of flexion, extension, and abduction are lost with humeral neck level amputation, shoulder disarticulation, and forequarter amputation. They are usually amputations related to malignancy and severe trauma in which no distal level amputation was possible. These levels of amputation present challenges to achieving adequate suspension and functional use of the prosthesis. Newer myoelectric techniques are gaining ground in achieving the multijoint control that is needed in optimal prosthetic restoration for these very proximal upper limb amputations.
Principles of Limb Salvage and Amputation Surgery
Limb Salvage
Injury scores were developed for severe trauma-related limb injuries, to help determine which vascular injury patients would benefit from primary amputation versus an attempt at limb salvage. Their validity has been questioned. The mangled extremity syndrome is defined as significant injury to at least three of the four tissue groups (skin/soft tissue, nerve, vessel and bone).24 The mangled extremity scoring systems have been shown to be poor predictors of amputation or salvage with regard to functional outcome.18,39,48 Ly et al.37 concluded that the available injury severity scores are not predictive of functional recovery of patients who undergo reconstruction surgery. Bosse et al.,6 using the Sickness Impact Profile, presented evidence that the functional outcomes from limb salvage and reconstruction after severe trauma were the same at 2 years for those who underwent amputation. Finally, in this salvage-versus-amputation equation, no significant long-term psychological outcome advantage has been reported for limb salvage surgery compared with amputation.45 Consequently, objective measures have not functionally supported the natural desires of the patient and the tendency of the trauma team to make all attempts at salvaging the limb.
In severe limb trauma that includes defects from burns and tumor resection, the appropriate soft tissue restoration is an essential component of the overall treatment. This is common both to limb salvage and amputation, especially when critical lengths are being preserved. It requires a vascularized flap that can protect the neurovascular and musculotendonous structures (Box 12-3). The pedicle flap is one where a local muscle inclusive of the overlying skin is moved over with it own blood supply to fill a large defect. A microvascular free flap is one in which the donor tissue is taken from a different site and the microvasculature of the donor tissue is anastomosed to the available vessels in the site of the defect. The feasibility of limb salvage is determined partly by the ability to reconstruct the soft tissue defect. In the upper limb, few pedicle flap options are available to repair significant defects. The recent advancement of microvascular reconstruction techniques and free flaps from sites like the rectus abdominus have promoted the option of limb salvage and preserved limb length.
BOX 12-3 Skin Flaps
For those with malignant tumors, 70% to 85% are treated by limb salvage without compromising the oncologic result.50 The goal of this type of surgery is to preserve function, prevent tumor recurrence, and enable the rapid administration of chemotherapy or radiation therapy. In the tumors of the hand, ray resection is done. In the wrist, multiple options are available such as an endoprosthesis implant or an allograft or vascularized bone transplant (e.g., fibula). For the elbow an endoprosthetic reconstruction is the best possible option. The humerus is similar to the wrist in that an endoprosthesis, or an allograft, or a vascularized bone transplant can be used. For tumors of the scapula or proximal humerus, a forequarter amputation or flail arm is prevented by reconstruction with a combination of an endoprosthesis and allograft. These types of reconstruction would not be possible without major improvements in radiography, chemotherapy, radiotherapy, and staging.50
The complication rate is much higher after limb salvage than after amputation in the oncology population. These complications can be divided into early and late. The earliest complications include infection, wound necrosis, and neurapraxia. The late complications include aseptic loosening, prosthetic fracture and dislocation, and graft nonunion.4 Consequently additional surgery is often necessary. Advancements in resection techniques, radiation, and chemotherapy have improved both functional limb survival and life expectancy. Serletti et al.,45 using the Enneking Outcome Measurement Scale, reported the functional outcome as “excellent” or “good” in greater than 70% of the patients who had reconstruction after resection of limb sarcomas. The Enneking Outcome Measurement Scale is an outcome tool that assesses seven characteristics of upper limb use: ROM, stability, deformity, pain level, strength, functional activity, and emotional acceptance. Limb salvage has cosmetic advantages, but whether the quality of life of these patients is superior to that of those who undergo amputation is unclear.
Reimplantation of traumatically amputated limbs is now possible, especially in children, because of the potential for successful neurologic recovery.30 Effective treatment of the patient and the ischemic, detached body part requires appropriate early cooling and prompt reimplantation within the initial 12-hour window. Predictors of successful reimplantation include adequate preservation, contraction of the muscle in the amputated limb after stimulation, the level of injury, and no tobacco use. The best predictor of success is the serum potassium level in the amputated segment. If the serum potassium level is higher than 6.5 mmol/L, reimplantation should be avoided.51
Reimplantation is indicated in levels from the distal forearm to the fingers. The more proximal to the wrist, the greater the amount of ischemic muscle mass and the more complex the metabolic and surgical demands. Approximately 85% of replanted parts remain viable. Sensory recovery with two-point discrimination occurs in 50% of adults.35 The functional results are more promising in children, but the viability rate is lower because of the technically demanding microvascular surgery. Major limb reimplantation entails significant metabolic disturbance and risk. It requires scrupulous medical management. Reimplantation is contraindicated in those with crushed and mangled limbs and those with atherosclerosis. Because nerves transected in the proximal arm must regenerate over a considerable length, only limited motor return is typically seen in the forearm and hand. Useful function of the wrist and hand is unusual and limited at best. Function can often be improved by converting these patients to transradial prosthetic wearers. Unfortunately it means performing a transradial amputation after successful transhumeral reimplantation. This is known as segmental reimplantation, in which portions of compromised limbs are salvaged that would otherwise have been discarded.
During the past 10 years, collaboration between hand surgeons and immunologists has led to successes in hand transplantation. Recent advances learned from clinical organ transplant immunosuppression known as composite tissue allograft (CTA) have permitted hand transplantation to progress beyond the first one done in the United States in 1997. CTA is the term used to describe transplantation of multiple tissues (skin, muscle, bone, cartilage, nerve, tendon, blood vessels) as a functional unit. In addition to the usual problems of identifying an organ donor, selecting a donor for a hand transplant must involve additional and careful emphasis on matching skin color, skin tone, gender, ethnicity and race, and the size of the hand. After a hand has been lost, much time can pass before a donor is found. Representation of the hand in the individual’s brain is lost because of cortical reorganization during this time. Researchers have learned through functional magnetic resonance imaging that after transplantation, amputation-induced cortical reorganization is reversed to reestablish the hand “image.”5 During transplantation the surgeon repairs the tissues in the following order: bone fixation, tendon repair, artery repair, nerve repair, and vein repair. The surgery can last from 12 to 16 hours, almost double that of heart and liver transplants. Immunosuppression after CTA is composed of two elements: treating the patient with monoclonal antibodies on the day of transplant, followed by a donor bone marrow infusion several days later. Typical postoperative complications include vessel thrombosis, infections, and rejection. Rejection can appear as a spotty, patchy, or blotchy rash. It could appear anywhere on the transplant and is usually painless. As rejection appears first in the skin, the clinical team and patients are encouraged to carefully watch for the signs. Unlike internal organ transplants, where rejection is difficult to detect early, it is relatively easy to monitor in the hand, allowing for early biopsy and treatment. Recovery is relatively slow, requiring an extensive program of occupational therapy. As of this printing the longest reported patient follow-up has been 43 months. This patient, a paramedic instructor, is now reportedly able to start intravenous lines and perform endotracheal intubation.
Amputation
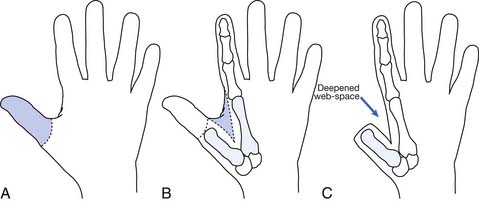
Hand function is vital in our competitive and industrialized society. There are many techniques for reconstruction of the hand. It is much better to have a painless hand with some grasp function and sensation preserved than to have a prosthesis. The most important part of the hand is the opposable thumb. The goal is to preserve as much of the sensate thumb as possible. Phalangization (Figure 12-3) of the metacarpals is a reconstructive technique in which the web space is deepened between the digits to provide more mobile digits. This works well for the thumb especially if the first metacarpal is adjusted to create opposition to the thumb. Pollicization (Figure 12-4) is the process of moving a finger with its nerve and blood supply to the site of the amputated thumb. This allows fine and gross grasp through opposition. A prosthesis for a hand amputation is inferior to the functional outcomes achieved with reconstructed hands.9 In reconstructing the hand, three issues should be considered: (1) preservation of sensitivity to the grasping surface; (2) the consequences of scarring; and (3) cosmetic acceptability.
Wrist disarticulation involves removal of the radius and ulna to the styloid processes, because there is no benefit to retaining the carpal bones. It retains the distal radial-ulnar joint, preserving more forearm rotation. The prosthetic attachment to the bulbous end is enhanced if the distal radial flare is retained for suspension. Burkhalter et al.10 indicate that it is important that the radial and ulnar styloids be resected slightly to minimize the discomfort the amputee will experience in active supination and pronation within the prosthetic socket. Tenodesis of the major forearm muscles stabilizes these groups and improves functional outcome, including myoelectric performance. Pronation and supination, as well as full elbow motion, are preserved with wrist disarticulation. Some will argue that (1) the wrist disarticulation creates a complicated prosthetic situation with difficult socket fabrication; (2) conventional wrist units are too long and cannot be used; and (3) it is harder to fit with a myoelectric prosthesis because there is no room to conceal the electronics and power supply.
Transradial amputation involves the myodesis of the forearm muscles and equal volar and dorsal skin flaps for closure. It is extremely functional, with forearm rotation and strength that is proportional to the length retained. The shorter the transradial amputation, the more the elbow and humerus are needed for suspension. Preserving the elbow joint is paramount because of the functional outcome possible with prosthetic enhancement. If the amputation must be very proximal, then an ulna 1.5 to 2 inches long is still adequate to preserve the elbow joint. To fit this very short residual limb with a prosthesis, it might be helpful to detach the biceps and reattach it to the ulna.36
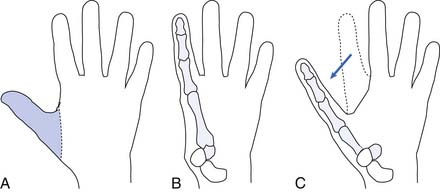
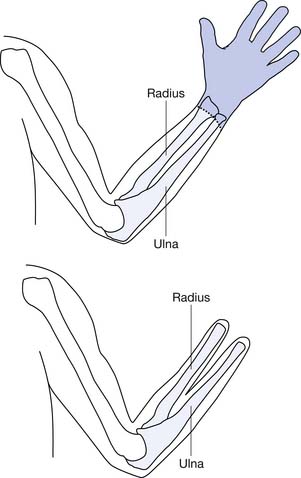
A couple of special situations arise with transradial amputations. One is when the forearm bone is considerably longer than the other and the longer bone can be covered with an adequate soft tissue envelope. Rather than decrease prosthetic function by shortening the longer bone, it may be preferable to create a one-bone forearm. Another is the Krukenberg amputation (Figure 12-5), which transforms the residual ulna and radius into digits that have significant forceful prehension and retained ability to manipulate because of preserved sensation. This is an option for patients who have at least 4 inches of residual limb, those with bilateral amputation, and those with limited prosthetic facilities. It can be fitted with conventional as well as myoelectric prostheses.
Elbow disarticulation allows the transfer of humeral rotation to the prosthesis through the myodesis of the biceps and triceps, and it preserves a stronger lever. Although the skin flaps are approximately equal, the posterior muscle flap remains longer than the anterior muscle flap, to wrap around and cushion the end of the humerus. The ultimate position of the scar is not critical with modern total-contact sockets, but beware of the vulnerable skin over the medial epicondyle. The full humeral length precludes the use of a myoelectric elbow. Elbow disarticulation causes some prosthetic fitting challenges because the outside “elbow” hinge creates a bulky limb that is longer and asymmetric compared with the opposite limb. Disarticulation is the level of choice for juvenile amputees. The high incidence of residual limb revision because of bony overgrowth is avoided, and humeral growth is preserved. It remains controversial who is a good candidate for elbow disarticulation, but modern prosthetic fabrication techniques can overcome the socket and cosmetic difficulties.12,14
Transhumeral amputations are performed at or proximal to the supracondylar level. The humerus is sectioned at least 3 cm from the joint to allow for fit of the prosthetic elbow mechanism. Transhumeral amputations should be performed with minimal periosteal stripping to prevent the occurrence of bony spurs. Rough edges should be removed, but beveling of the bone is unnecessary. All possible length should be preserved to transmit glenohumeral motions through the prosthesis. To help preserve humeral length, at times it should be considered whether free-flap coverage and skin graft coverage are possible alternatives to allow primary closure. The anterior and posterior fascias over the flexor and extensor muscle groups are sutured together to cover the end of the humerus. Biceps and triceps myoplasty preserves strength for prosthetic control and myoelectric signals. Myodesis is rarely needed.38 Performing a more proximal amputation at the level of the surgical neck, which is the site of insertion of the pectoralis major, results in the same function as if a shoulder disarticulation had been done. This is because independent motion of the humerus is no longer possible. However, because the terminal device is controlled by active shoulder girdle motion, the humeral head should be preserved when amputation has to be done proximally.
Acute Management: Preamputation Through Early Rehabilitation
Acute Postamputation
This phase begins with an understanding that the decision to amputate is emotionally powerful for the patient, family, and clinical team. Amputation is not a failure but rather reconstructive surgery that creates improved functional possibilities and resumption of one’s life. The focus of the immediate postamputation period is to control pain and edema, promote wound healing, prevent contractures, initiate remobilization, and continue the supportive counseling and education (Box 12-4). This must be individualized to meet the needs of each patient. Surgical site infection needs to be seriously considered when pain, drainage, and edema are increasing despite the reasonable control measures instituted. The earlier an infection is eradicated, the earlier the time-sensitive prosthetic phase can begin. The goal for rehabilitation is for patients to acquire the skills and equipment needed to achieve prosthetic acceptance and holistic reintegration back into their own lives. It is imperative that the prosthesis be introduced at the earliest possible time after amputation.
Pain control requires an early, aggressive approach that considers the multiple potential pain generators in the postsurgical period. The patient-controlled analgesia systems are often the first-line treatment by the surgical team. This is transitioned to regularly scheduled long- and short-acting oral narcotic medications. It is imperative to maintain consistent pain control. Loss of adequate pain control is painful for the patient and disrupts the timely pursuit of the rehabilitation program. The escalation of the doses of opiates needs to be avoided, if possible, by addressing other pain generators. Understanding the characteristics of postsurgical residual limb pain and phantom pain allows the clinical team to choose pain interventions wisely. Residual limb pain is located in the remaining limb and generated from the soft tissue and musculoskeletal components. Phantom limb pain is pain in the absent limb and is considered neuropathic.17 The nonsteroidal antiinflammatory drugs and nonopiate pain relievers are helpful, and these can diminish the need for higher doses of opiates. Opiates administered at safe doses are often ineffective against phantom pain. Careful attention should be given to the description, timing, and quality of the pain complaint to tease out the central neuropathic pain component inclusive of painful phantom sensations versus peripheral nerve–generated pain. Peripheral nerve pain is more intense at night, and it is characterized as burning, stabbing, and buzzing. Phantom sensations occur in greater than 70% of amputees and do not have to be treated unless painful and disruptive. The use of medications known for controlling neuropathic pain and sensations, such as some anticonvulsants and antidepressants, can also diminish the need for opiates.
The new amputee should be taught how to change the dressings and self-administer the desensitization techniques. Desensitization techniques help to eliminate the hypersensitivity to touch. They include compression, tapping, massage, and application of different textures. These techniques are performed for 20 to 30 minutes three times per day as tolerated by the skin and scar.22 The use of modalities such as transcutaneous nerve stimulation, heat, and cold are also useful adjuvants for pain and diminish opiate need. Ramachandran and Rogers-Ramachandran43 have reduced phantom pain using mirrors to visually trick the brain. Because the loss of a limb is emotionally “painful,” the team should address and acknowledge this. It should be kept in mind that from the individual’s psychological standpoint, it might be more socially acceptable to express the psychological pain in terms of generalized pain complaints. It is important to address the psychological pain early, through grief counseling, peer visitation, and education.
Edema control begins once the last suture or staple is placed by the surgeon. If there is no contraindication and the surgeon has the appropriate training, an immediate postoperative rigid dressing (IPORD) can be placed in the operating room. This is a special cast placed on the residual limb by the surgeon or certified professional. The control of edema leads to earlier wound healing and improved pain control through the reduction of pain mediators in the accumulated “third-spaced fluid.” Typically additional shrinkage of the residual limb occurs after the initial IPORD placement, necessitating its early replacement. The rigid dressing can be removed in 5 to 7 days and replaced with a fresh cast. The attachment of joints and a terminal device to this rigid dressing creates an immediate postoperative prosthesis that can allow early functional use of the residual limb. The IPORD is the preferred treatment approach for the transradial amputation, and if healing progresses without issue, the second cast can be replaced with the first prosthesis.19
The formation of heterotopic bone impairing joint function and ROM should be considered in these complex trauma cases and can be diagnosed with the help of laboratory testing and triple-phase bone scan. The treatment of heterotopic ossification beyond trying to maintain ROM is limited, and surgical intervention is not feasible until the heterotopic bone matures at approximately 12 to 18 months after injury.23 Proper limb positioning and frequent monitoring of joint mobility are necessary. Any loss of ROM in a joint of the residual limb can have significant effects on functional use of the prosthesis. The loss of ROM needs to be investigated and aggressively managed to maximize range.
Preprosthetic training begins with the early postsurgical therapy visit and continues until prosthetic fitting is completed. Prosthetic fabrication and fitting ideally should be completed within 4 to 8 weeks after surgery. Early prosthetic fitting is important, because prosthetic acceptance declines if fitting is delayed beyond the third postoperative month.20 Preprosthetic training is critical to maintain motivation and create an easier transition to prosthetic use. Amputation results in a loss of body symmetry. This imbalance results in shoulder elevation and scapula rotation on the affected side, as well as loss of neutral positioning of the residual limb. Close attention must be paid to the individual’s awkward or compensatory body motions when approaching an object. The rebalancing begins with observing and correcting static postures in the mirror. The mirror remains an important tool in conscious recognition and correction of the abnormal positioning. The amputee is encouraged to use muscle memory to relearn correct postural and limb positioning control.22 As remobilization progresses, emphasis is placed on recognizing the abnormal postures and positioning that occur with basic activities of daily living (ADLs).
ADLs are mastered with one hand and, when appropriate, with the use of adaptive equipment. The amputee progresses from independence with basic hygiene to the advanced homemaking tasks. Hand dominance is retrained when necessary, especially with handwriting and keyboarding. Repetitive tasks can be used for strengthening. These tasks include fine motor exercises with nuts and bolts or tweezers, as well as gross motor exercises with equipment and mirrors. Proprioceptive neuromuscular facilitation is a particularly effective approach that enables the therapist to work in diagonal planes, vary the amount of resistance, and concentrate on specific areas of weakness. Isometric exercises are effective in creating muscle bulk for stabilization of the arm in the socket of the prosthesis. The stability of the prosthesis depends on both the bulk of the stabilizing musculature and the amputee’s ability to voluntarily vary residual limb configuration. Because balance is often disrupted in a new amputee, the goals should include strengthening of the trunk, core, and lower limbs using isometric exercise and aerobic training. Depending on level of loss, the upper limb amputee should begin to practice several motions that will be needed to control the prosthesis (Box 12-5).
BOX 12-5 Specific Movements Necessary to Control a Prosthesis and Maintenance of Range of Motion
Specific Movements Necessary to Control a Prosthesis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree