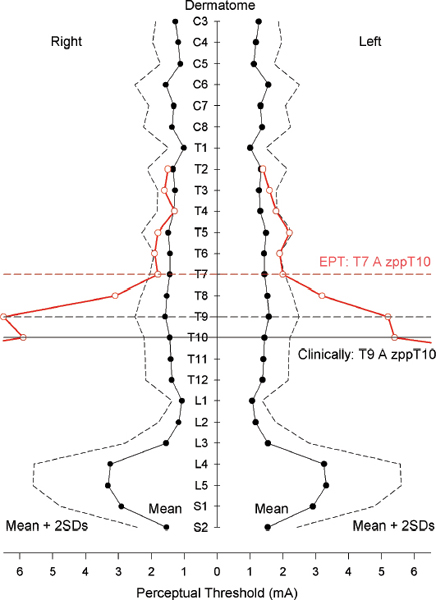
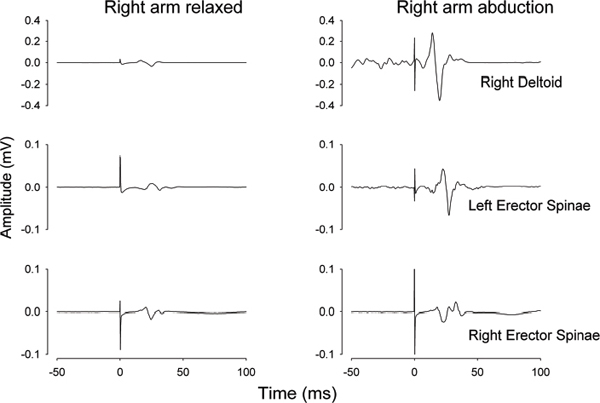
39 Key Points 1. Quantitative and objective electrophysiological tests of sensory, motor, and autonomic function are reviewed as adjuncts to the International Standards for Neurological Classification of Spinal Cord Injury (ISNSCI) and the American Spinal Injury Association Impairment Scale (AIS) neurological assessment. 2. The merits of the electrical perceptual test of cutaneous sensibility and the use of dermatomal sensory evoked potentials are assessed and compared. 3. The use of noninvasive electromyographic recording of muscle function and the ability to test the patency of the corticospinal tract with transcranial magnetic stimulation is reviewed. 4. The use of a specific test of autonomic function in SCI, the sympathetic skin response, is described as having the potential to provide insight into the supraspinal access to the sympathetic chain. A limited number of clinical trials for the repair of spinal cord injury (SCI) are under way, with additional trials based on novel therapies being widely anticipated from successful preclinical studies. One integral aspect of the design of clinical trials for SCI is identifying the type and breadth of the primary and secondary outcome measures.1 This chapter is devoted to discussing several novel and improved techniques for ascertaining the level and completeness of an SCI. Although the general goal of a clinical trial will have been an improvement in functional outcome, and hence anticipated, the actual outcome will inevitably be uncertain and may even be adverse. Outcome measures for physiological systems should therefore be based on methods that allow both for improvements and for worsening of the condition. Additionally, the methods employed as outcome measures should be capable of detecting small physiological changes that could be limited to change at a single vertebral level of the spinal cord. Preclinical studies of novel therapies designed to promote regeneration of spinal cord axons indicate that regenerating axons may descend or ascend the cord for only a few centimeters.2 Translated to humans, this amount of potential reinnervation would result in renewed or new functional connections restricted to one or two vertebral levels. The current gold standard for clinical assessment of SCI is the American Spinal Injury Association (ASIA) Impairment Scale (AIS), a set of standard neurological classifications that comprises tests of sensory and motor function. There are several limitations to the AIS assessment. First, sensory cutaneous evaluation of each dermatome is scored simply as either normal, absent, or abnormal sensation. Abnormal sensation currently includes both heightened and lowered sensitivity as well as allodynia. Second, motor assessment is confined to the myotomes of the four limbs and ignores the trunk. A recent improvement has been the recommendation to score the upper and lower limbs separately.3 Furthermore, the minimal clinically important difference is generally regarded as not having been established for either sensory or motor AIS assessments.4 There is no component of autonomic assessment in the ASIA standard neurological classification of spinal injury. The only references to visceral function are questions on voluntary anal contraction and anal sensation. It is currently recognized that bladder and bowel require functional testing and that sexual, cardiac, vasomotor, and sudomotor function should be addressed.5 The following sections represent physiological tests that have been developed or improved upon in the Clinical Initiative funded by the International Spinal Research Trust (ISRT) (http://www.spinal-research.org).6 At the time of an assessment, subjects may have lived for months or years with a disability caused by their SCI. Their impression of what constitutes normal sensibility for a dermatome may well have changed with time. Any test that is objective, quantitative, and does not rely on reporting the quality of sensation would augment the limited AIS sensory grading. Methods that fit this description are frequently bracketed under the description of quantitative sensory testing (QST).7 Two electrophysiological techniques that also provide quantitative and objective measures of somatosensory function are the electrical perceptual threshold (EPT) test and somatosensory evoked potentials. The EPT was developed for the assessment of cutaneous sensibility8 and later validated against the AIS sensory grading.9 EPT measures the threshold, or minimally detectable sensory appreciation, of constant-current square-wave pulses applied to the AIS test point of a dermatome. EPT determinations for subjects with SCI may be superimposed on a normal template constructed from the results of up to 40 neurologically normal individuals (Fig. 39.1). EPT values were found to lie outside the normal range below the level of injury defined clinically by the AIS sensory grading.9 However, Savic et al. also reported a large number of abnormal EPT readings at the level of injury (i.e., the most caudal clinically normal dermatome), and even up to two or three dermatomes higher (Fig. 39.1), indicating that the ASIA clinical assessment had placed the level of injury too low.9 The discrepancy was tentatively attributed to adaptation over time to sensations elicited by cutaneous stimulation of dermatomes affected by the injury. The EPT has since undergone repeatability evaluation for both inter- and intrarater trials in SCI10 and compared different stimulators. Good intra- and inter-rater reliability for the EPT has now been confirmed11 in control subjects for the C3, T1, L3, and S2 dermatomes. The EPT is most likely testing the state of the posterior (dorsal) column pathway. In support of this, it is generally accepted that low-amplitude and short-duration current pulses applied to the skin will preferentially excite nerve endings or axons of large Aαβ myelinated fibers that convey innocuous modalities of cutaneous sensation, which agrees with reports of subjects that EPT test pulses are perceived as a light tapping sensation. Also supporting the association is the observation that some individuals with incomplete SCI lack any pain perception to large current pulses (> 10 mA) but have normal EPT values for certain dermatomes below the level of the lesion.12 Similarly, a patient (T2 AIS D) with anterior spinal artery infarction has been reported with preserved light touch sensation and proprioception but abnormal pinprick discrimination consistent with an anterior cord syndrome, and EPT values within the normal range.11 Fig. 39.1 Electrical perceptual threshold (EPT) results from a subject with complete spinal cord injury (open circles, red line) superimposed on the normal template (solid circles, black line). The clinical level of injury (American Spinal Injury Association Impairment Scale sensory) was T9 with a zone of partial preservation to T10. The EPT reveals the most caudal normal dermatome to be T7. (From Savic G, Bergstrom EMK, Frankel HL, Jamous MA, Ellaway PH, Davey NJ. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord 2006;44:560–566. Reprinted with permission.) In summary, EPT provides an adjunct to the AIS for sensory function by being a more quantitative and more objective measure. The method has good inter- and intrarater reliability. It is likely that EPT tests the posterior (dorsal) column pathway for light touch and proprioception rather than the anterolateral spinothalamic tract. The clinical neurological assessment of SCI may be augmented by employing somatosensory evoked potentials (SSEPs). Such neurophysiological evaluation of the sensory pathways from the skin has provided greater accuracy in determining the level and extent of an injury and has been correlated with functional outcomes.13 The technique assesses the posterior (dorsal) column pathways of the spinal cord. A refinement of the technique is the dermatomal somatosensory evoked potential (dSSEP), which relates to a specific spinal segment. Rather than place the peripheral stimulating electrodes over a large nerve, electrodes are applied to the skin of a specific dermatome. The resulting evoked potentials are usually smaller in amplitude and lack the definition of SSEPs evoked by stimulation of a large nerve. However, the advantage is that dSSEPs may determine the level and extent of a spinal cord lesion with greater accuracy. Validation of the EPT has been provided against dSSEPs.12 Above the level of a spinal cord lesion, the dSSEP and EPT were comparable to those of control subjects. Close to the level of lesion, the dSSEP appeared abnormal and was of longer latency, and the EPT was raised above control values. Below the lesion, when the dSSEP was abolished, the EPT was raised further or unobtainable. In conclusion, the requirement to improve on the insufficient sensitivity of the AIS sensory assessment1 may be met by either the EPT or dSSEP methods. Either or both appear sufficiently sensitive as an adjunct to the AIS sensory assessment to detect change over individual segments and so provide assurance in terms of both safety and quantitative evidence that an intervention is working in a beneficial manner in early phase 1 or 2 clinical trials. Applying these techniques to measure cutaneous sensibility above and below the level of a lesion during trials could also point to the neurophysiological basis of the mechanism of change. An intervention may well induce changes in spinal cord circuitry as a result of sprouting or regeneration but additionally induce secondary plastic changes. These may occur above as well as at and below the lesion. EPT and dSSEPs have the potential to identify such change and, by indicating the basis of recovery, assist the refinement of any physical, pharmacological, or cell-based intervention designed to effect recovery of function.14 The current clinical gold standard for determining voluntary control of human motor function is the AIS assessment. This is limited to five key muscle groups of the upper limbs and five for the lower limbs. The trunk is not currently assessed. Moreover, the scores for the upper and lower limbs tend to be combined, presenting an overall score that, by itself, cannot provide a clear picture of the nature and extent of the injury. Electromyographic (EMG) investigation provides direct, quantitative, and objective insight into the integrity of the principal pathway involved in executing voluntary skeletal muscle activity, the corticospinal tract. The EMG approach that directly addresses the function of the corticospinal pathway is the use of transcranial magnetic stimulation (TMS) of the motor cortex. There are numerous reviews of the TMS technique and applications to the investigation of several neurological conditions,15 and TMS has frequently been used to provide motor outcome for SCI.16–19 Measures of the threshold, latency, and size of motor evoked potentials (MEPs) to TMS have been made longitudinally during natural recovery in SCI. These studies have failed to show a direct link between MEP parameters and functional or clinical recovery.20,21 In contrast, recovery of MEPs has been closely linked with functional recovery in response to weight-assisted tread-mill training in SCI.22 The observations of impaired facilitation of MEPs to voluntary effort in subjects with incomplete SCI are likely to be of significance in developing the TMS technique as an outcome measure for recovery of corticospinal tract function.23 A further application that would contribute greatly to motor assessment is the potential for TMS to be used with trunk muscles (Fig. 39.2). MEPs may be recorded from abdominal muscles, paravertebral muscles,24 and respiratory muscles.19,25 Moreover, the technique is able to provide insight into the ipsilateral projections of the corticospinal tract.26,27 The brain motor control assessment (BMCA)28 provides a quantitative analysis of surface EMG recordings during voluntary movement based on a voluntary response index (VRI). The method also has the potential to extend the limited range of muscles included in the AIS assessment, in addition to meeting the criteria for a more quantitative and objective measure. Usefully, the facility for TMS to demonstrate connection between the motor cortex and spinal motor neurons in persons with SCI has been related to the quality of postinjury voluntary motor control as assessed by the VRI.29 Finally, the inclusion of TMS for provision of quantitative outcome measures in SCI is supported by its being a noninvasive technique that is well tolerated by subjects. A recent review of risks associated with conventional TMS protocols reports few side effects and provides guidelines with respect to safety.30 The autonomic nervous system controls disparate body functions and also interacts closely with somatosensory systems and the central nervous control of voluntary movement. Apart from the innervation provided by cranial nerves, autonomic control is potentially affected by SCI at cervical, thoracic, lumbar, and sacral levels. Routine measurements of blood pressure and heart rate and questionnaires relating to bladder, bowel, and sexual function are important in determining the impact of an SCI on the autonomic nervous system. Detailed assessment, however, may require elaborate functional and physiological tests. Those tests may be impractical when repeated, and quantitative estimates are required for monitoring change in response to treatment on a clinical trial. A test that could be considered when cost, time, and patient acceptability are of concern is the sympathetic skin response (SSR). Fig. 39.2 Motor evoked potentials (MEPs) recorded from deltoid and erector spinae muscles of a control subject in response to transcranial magnetic stimulation (time zero) of the motor cortex. (Left) MEPs recorded with both arms relaxed and (right) with the right arm abducted. Note the greater facilitation of the left compared with the right erector spinae MEP during arm abduction. Unaveraged, single recordings. (Unpublished records)
Quantitative Tests of Sensory, Motor, and Autonomic Function
 Cutaneous Sensory Function
Cutaneous Sensory Function
Electrical Perceptual Threshold
Somatosensory Evoked Potentials
 Motor Function
Motor Function
Transcranial Magnetic Stimulation
 Autonomic Function
Autonomic Function
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


