Chapter 18 Pulmonary Medicine
Lung Disease in Primary Care
Smoking and Other Risk Factors for Lung Disease
The Centers for Disease Control and Prevention (CDC) describe smoking as the single most preventable cause of premature death in the United States, contributing to more than 400,000 deaths per year, or one in every five deaths. People who smoke suffer more than a 20-fold increase in risk of death from lung cancer and a 10-fold increase in risk of death resulting from bronchitis or emphysema (CDC, 1993). For women in the United States, there are more deaths caused by lung cancer than by breast cancer. Worldwide there were 5 million deaths attributable to smoking in 2000, almost 2 million of which were related to lung cancer and other lung diseases. WHO projects a doubling of smoking-related deaths by 2020 (Ezzati, 2003).
Even simple physician advice to quit smoking provides a marginal benefit of 2.5% of patients quitting successfully (Lancaster and Stead, 2004). Interventions that combine counseling plus education or group strategies plus pharmacologic treatment with nicotine replacement or specific medications can achieve sustained quit rates of 25% to 30% (Hughes et al., 2004; Solomon et al., 2005; Stead and Lancaster, 2005). The Agency for Healthcare Research and Quality (AHRQ, 2008) has provided a comprehensive update to previous clinical guidelines for smoking cessation and treating tobacco dependence. Counseling is most effective when it includes both practical problem solving and social support. Pharmacologic treatment, such as nicotine replacement, bupropion, and varenicline, has proved effective but is most effective when combined with counseling or group programs. The Legacy Foundation reports that ex-smokers have an average of eight quit attempts before ultimately sustaining a tobacco-free lifestyle. Box 18-1 defines a strategy for helping patients to quit smoking by a simple mnemonic of “five As” (ask, advise, assess, assist, arrange).
Box 18-1 The Five As: Strategies to Help Patients Quit Smoking
From Global Initiative for Chronic Obstructive Lung Disease: Executive Summary: Global Strategy for the Diagnosis, Management, and Prevention of COPD. Updated 2008. http://www.goldcopd.com/download.asp?intId=504.
Diagnostic Tools in Pulmonary Medicine
History and Physical Examination
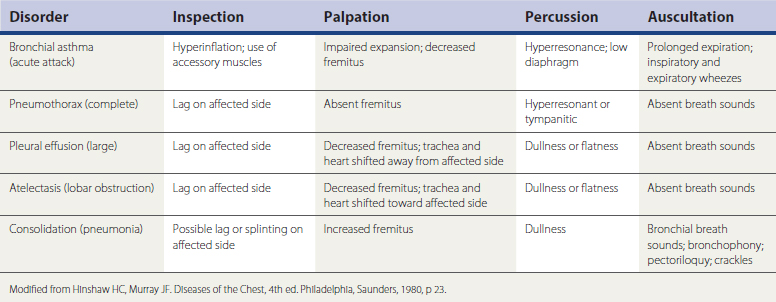
Palpation can identify thoracic wall abnormalities, mass lesions, or tenderness and show asymmetry of chest wall expansion. Percussion of lung fields should normally be resonant. Dullness to percussion can indicate fluid in the pleural space or consolidation of the lung itself, with fluid filling the normally air-filled alveolar spaces. Both these conditions also produce decreased breath sounds over the affected area. Hyperresonant lung fields on percussion can indicate the hyperexpansion of obstructive lung disease or even pneumothorax.
Signs of bronchial inflammation, mucus, and obstruction in the bronchial tree include coarse crackles and wheezes (sonorous or musical rhonchi). Additionally, in normal vesicular breathing, the duration of the inspiratory phase is longer than the expiratory phase—typically 90% of the expired air is exhaled in the first second. Prolongation of the expiratory phase is an early sign of obstruction, even before wheezing develops. The diagnostic implications of these physical examination findings are summarized in Table 18-1.
Pulmonary Function Testing
Previous office spirometry units were bulky and required frequent recalibration, but modern units are small, computerized, and often self-calibrating. Quality of testing is important. The patient should be seated comfortably and instructed appropriately. An adequate PFT must include three valid measures (quick start, good effort, maintenance of forced expiration for at least 6 seconds with no cough) and three relatively similar results (FVC varying by <200 mL).
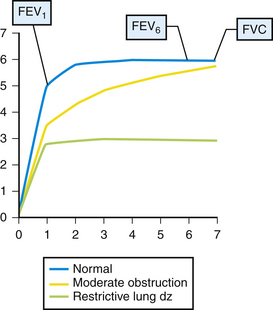
Results of office spirometry are presented both graphically and numerically, with actual values compared to values predicted by the patient’s age, height, and gender. Figure 18-1 shows the points at which two critical values, forced expiratory volume at 1 second (FEV1) and forced vital capacity (FVC), are measured on the time-volume curve. Figure 18-2 shows a typical flow-volume loop for patients that compares normal PFTs with obstructive lung disease and restrictive lung disease (Zoorob et al., 2002). Increasingly, the 6-second end point, or FEV6, is being used as a reliable and reproducible surrogate measure that can replace FVC for patients unable to complete forced expiration beyond 6 seconds.
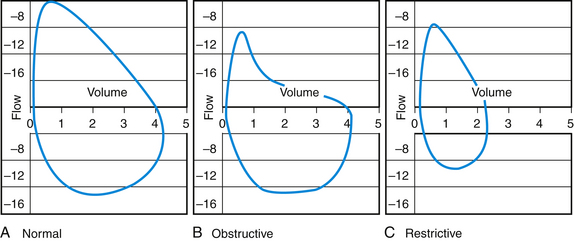
Figure 18-2 Flow-volume loop showing curves for normal (A), obstructive (B), and restrictive (C) lung disease.
Chest Radiography and Other Diagnostic Imaging
Despite major advances in imaging technology, the chest x-ray film (CXR) is still an important diagnostic modality that can clearly reveal signs of pneumonia, COPD, heart failure, tuberculosis, lung masses, and pleural effusion. Figure 18-3 shows a chest radiograph with right middle lobe pneumonia. Although the posteroanterior (PA) film shows some consolidation, it is attenuated by the overlying projection of a normal lower lobe. The lateral film, however, shows the classic wedge-shaped profile of a consolidated right middle lobe.
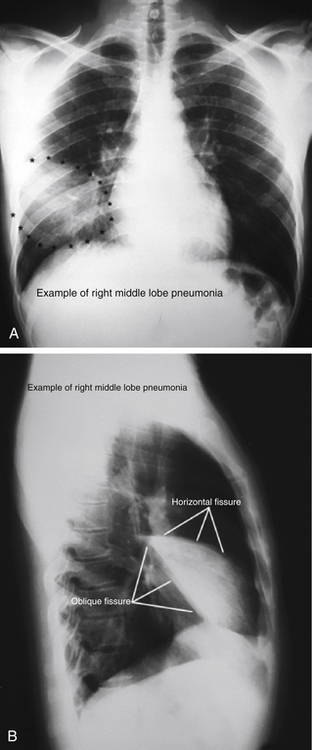
Figure 18-3 Posteroanterior (A) and lateral (B) chest radiographs showing right middle lobe pneumonia.
(From Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock. http://anatomy.uams.edu/anatomyhtml/xrays/xra_atlas39.html and http://anatomy.uams.edu/anatomyhtml/xrays/xra_atlas5.html.)
Chest radiography is also widely used in evaluating patients with suspected exposure to tuberculosis (TB), either because of symptoms, personal contact, or a positive intradermal purified protein derivative (PPD) test. Figure 18-4 shows the patchy infiltrates and hilar adenopathy typical of pulmonary TB, although TB can have a variety of presentations on chest x-ray films, including adenopathy, pleural scarring, infiltrates, cavitary lesions, and miliary TB.
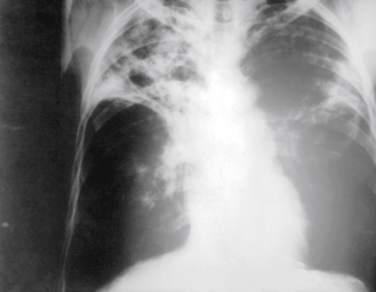
Figure 18-4 Chest radiograph showing tuberculosis, with upper lobe fibrotic patchy infiltrates and hilar adenopathy.
(From Centers for Disease Control and Prevention, Public Health Image Library, Image 2543. http://phil.cdc.gov/phil/home.asp.)
Positron emission tomography scans using 18F-fluorodeoxyglucose (FDG-PET) are becoming increasingly useful in evaluating lung cancers and lymphomas. The ability to define anatomy and metabolism, that is, glucose uptake within the tumor, makes the PET scan useful for staging, detecting node involvement, and defining resectability, tumor response to therapy, and tumor recurrence (Avril and Weber, 2005). Diagnosing solitary pulmonary nodules is a common challenge in primary care. A meta-analysis of studies comparing dynamic CT, magnetic resonance imaging (MRI), FDG-PET, and single-photon emission computed tomography (SPECT) scans based on positive and negative likelihood ratios for identifying malignant versus nonmalignant solitary nodules found that all four were similarly accurate (Cronin et al., 2008). Nuclear medicine studies such as gallium scans are also used extensively in evaluating pulmonary symptoms in patients with human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS) and are discussed in the section on HIV-related pulmonary infections in more detail.
Common Pulmonary Symptoms
Shortness of Breath
A common presenting symptom in pulmonary disease is shortness of breath. The fundamental question in patients presenting with recent-onset or episodic shortness of breath is this: Is it a lung problem, a heart problem, or something else? The most common pulmonary causes of chronic or repeated episodes of shortness of breath include asthma, smoking-related COPD, chronic lung infections (TB and HIV-related infections), and occupational pneumoconiosis. Acute-onset shortness of breath can be caused by acute exacerbations of any of these chronic conditions, by acute infections such as pneumonia or acute bronchitis, or by spontaneous pneumothorax. Among otherwise healthy children, shortness of breath can be related to asthma, bronchiolitis, pneumonia, or upper-airway problems such as croup or epiglottitis. Chronic shortness of breath in children can be related to poorly controlled asthma, bronchopulmonary dysplasia from infancy, or chronic diseases (e.g., cystic fibrosis).
Formal cardiac ECG stress testing by exercise treadmill can quantify the level of exercise tolerance and diagnose cardiac ischemia or angina-equivalent conditions, in which a person has ischemia-induced shortness of breath but no chest pain. However, the test has a sensitivity of only 63% and specificity of 74% (86% specificity in the setting of three-vessel or left main coronary artery disease) (Gibbons et al., 1997). Other types of cardiac stress testing, such as exercise echocardiography or the nuclear medicine thallium treadmill test, can be more specific (see Chapter 27) (Mayo Clinic, 1996). The American College of Cardiology and American Society of Echocardiography published a recent consensus guideline on the appropriate use of stress echocardiography for specific clinical scenarios (Douglas et al., 2008). For patients unable to exercise, increased cardiac work may be induced pharmacologically, but dipyridamole and adenosine can each cause bronchospasm and should be avoided in patients with asthma or any other obstructive lung disease or undiagnosed pulmonary conditions (Tak and Gutierrez, 2004).
Any suspicion of pulmonary embolism (PE) as a cause of acute shortness of breath requires specific diagnostic evaluation to allow quick intervention in this potentially life-threatening condition. Acute-onset shortness of breath coupled with pleuritic chest pain, hemoptysis, wedge-shaped pulmonary infarct lesions on chest radiograph, and an S1Q3 pattern and tachycardia on ECG can all point specifically to a diagnosis of PE, but most patients have a more nonspecific presentation. All patients with acute-onset shortness of breath with no apparent cause should be evaluated for PE. Physical examination for signs of deep venous thrombosis (DVT) (e.g., asymmetry in calf or thigh diameter, calf tenderness, Homans sign) are relatively insensitive and nonspecific, whereas other tests (e.g., D-dimer, CT angiography) can more accurately confirm the presence of significant underlying DVT. Clinical decision rules using objective scoring algorithms help establish pretest probability, which in turn enhances the predictive value of other tests for PE (Wells et al., 2000) (see later discussion).
Cough
Patients with HIV/AIDS or other compromise of the immune system deserve specific evaluation (see Chapter 17). HIV testing may be indicated in patients with any risk factors, because pulmonary symptoms can be the first manifestation of symptomatic HIV disease. In patients known to be HIV positive, tests in addition to chest radiography and HRCT could include gallium scan, PET, bronchoscopy with BAL for stains and cultures, PPD, and sputum testing for PCP.
Obstructive Lung Disease
The most common chronic lung diseases that have a major global impact on disability and health care costs are three obstructive lung diseases: asthma, chronic obstructive pulmonary disease, and chronic bronchitis. Some patients have features of more than one of these conditions, such as the patient with asthma (acute episodes of reversible obstruction) who also has chronic bronchitis (cough productive of phlegm at least 3 months of the year for at least 2 years in a row), or the adult patient with asthma who is developing some level of irreversible decline in pulmonary function. COPD alone can ultimately result in pathologic signs of emphysema, a diagnosis previously made only with tissue pathology or large blebs on x-ray film but increasingly visible with various multislice HRCT techniques.
Asthma
Clinical Presentation and Diagnosis
On auscultation, the earliest sign of airway obstruction is a prolonged expiratory phase (expiratory phase longer than inspiratory phase). A more obvious sign of asthma is expiratory wheezing. Wheezing can sometimes be brought out by forced expiration, performed by asking the patient to blow out forcefully while the examiner listens over the second intercostal space at the right costal margin. More severe cases of obstruction can result in both inspiratory and expiratory wheezing. In the most severe cases, wheezing might not be audible at all because airflow is minimal. In these cases, wheezing becomes much more prominent as airflow improves. Another sign of severity of an acute episode is pulsus paradoxus, defined as a decrease in systolic blood pressure of more than 20 mm Hg during inspiration.
Treatment
Chronic Care and Disease Management
To achieve optimal outcomes, each patient should have a personal asthma care plan, which can be summarized in the mnemonic MAP (Box 18-2). The management plan refers to daily medications or activities such as measuring peak flow; an action plan is needed for specific steps to take in the event of increased symptoms or deteriorating peak-flow values; and a prevention plan focuses on understanding personal and environmental triggers, such as avoiding passive exposure to cigarette smoke and eliminating dust mite and cockroach antigens.
Appropriate chronic care requires appropriate staging of the clinical severity of asthma. Stage 1 is intermittent and stages 2, 3, and 4 all represent persistent (mild, moderate, and severe) disease. Criteria for classification of patients into stages 1 to 4 are shown in Figure 18-5 (NHLBI, 1997). This staging of asthma is done based on the level and frequency of symptoms or airflow obstruction before beginning treatment. The patient’s step is determined by the most severe feature, and classification refers to symptoms before starting treatment. Pharmacologic treatment of asthma is linked to this classification, as shown in the treatment algorithm (Fig. 18-6).
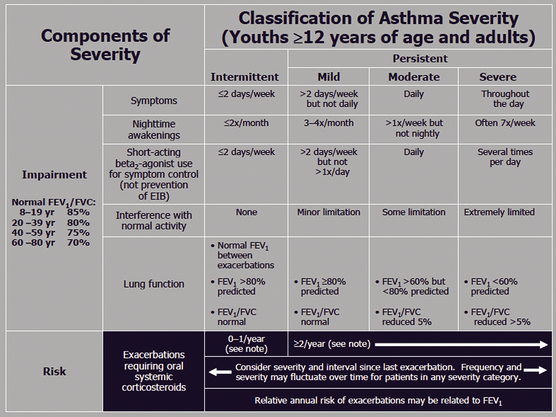
Figure 18-5 Classification of asthma severity in adolescents 12 years or older and adults.
(From Guidelines for the Diagnosis and Management of Asthma; National Asthma Education and Prevention Program (NAEPP) Coordinating Committee, 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
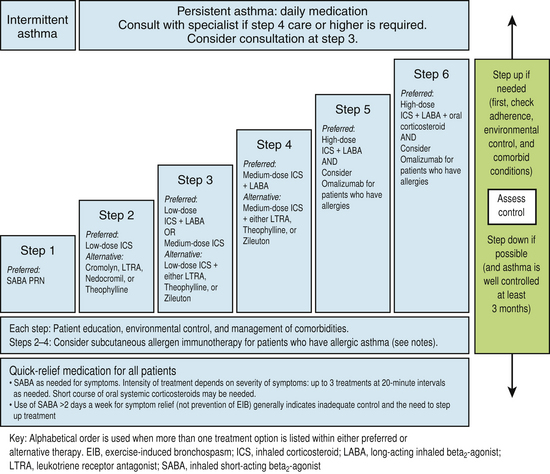
Figure 18-6 Stepwise approach for managing asthma in adolescents (≥12 years) and adults.
(From Guidelines for the Diagnosis and Management of Asthma; National Asthma Education and Prevention Program (NAEPP) Coordinating Committee, 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.)
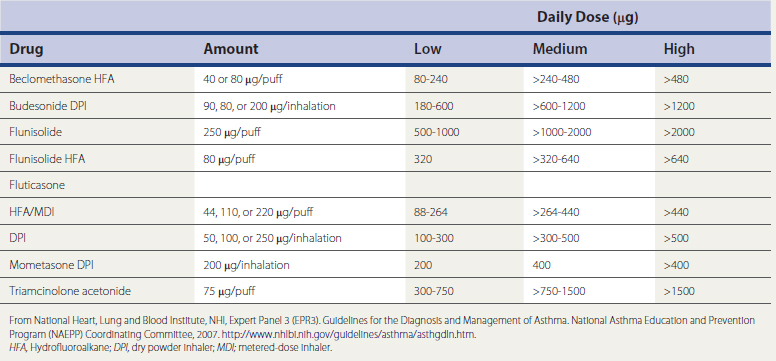
A meta-analysis found that inhaled corticosteroids reduced asthma exacerbations by 55% compared with placebo or short-acting β-agonists, and long-acting β-agonists (LABAs) reduced flare-ups by only 26% (Sin et al., 2004). Similarly, a Cochrane Database review found that inhaled steroids at a dose equivalent to 400 μg/day of beclomethasone are more effective than leukotriene antagonists, and that inhaled corticosteroids should be considered first-line monotherapy for persistent asthma. Another Cochrane review found that patients with mild to moderate disease achieve similar levels of asthma control taking low doses (≤200 μg/day) as high doses (≥500 μg/day) of fluticasone, and that side effects are greater with higher doses. A dose equivalency chart for inhaled steroids is shown in Table 18-2. A new approach to monitoring therapy by measuring the fraction of exhaled nitric oxide (FENO) can allow better adjustment of inhaled-corticosteroid doses in the future (Smith et al., 2005). High-dose inhaled corticosteroids are useful primarily in weaning patients from oral steroids.
Table 18-2 Estimated Comparative Daily Doses for Inhaled Corticosteroids for Adolescents (Age ≥12 Years) and Adults
KEY TREATMENT
Immunotherapy also appears to be effective. A Cochrane review found that allergen immunotherapy reduces asthma symptoms and use of asthma medications at a level similar to that of inhaled corticosteroids (Abramson et al., 2003a). A newer third-line therapy is the once- or twice-monthly injection of monoclonal anti-IgE antibodies (omalizumab), an expensive therapy that is associated with a 98% to 99% reduction in free IgE and significantly fewer exacerbations of asthma, even allowing some patients to be weaned from inhaled corticosteroids (Walker et al., 2003).
Chronic Obstructive Pulmonary Disease and Chronic Bronchitis
Epidemiology and Risk Factors
Smoking is the most important risk factor for COPD and causes ongoing damage in COPD patients, as measured by an accelerated decline in FEV1 compared with nonsmokers or ex-smokers. Among COPD patients who have quit smoking, exposure to secondhand smoke can also be a trigger factor for acute exacerbations. Variation in environmental air quality (ozone and small particulates) is also a factor associated with acute exacerbations of COPD. In many countries, air pollution can be a major source of smoking-equivalent damage to the respiratory tract in impoverished settings where daily cooking over indoor fires or charcoal is common. Other trigger factors for acute exacerbations include acute upper respiratory infections, sinusitis, exposure to dust or pet dander, and intercurrent illness. However, once patients reach a more severe stage of illness in which pulmonary reserves are minimal, almost any small change (e.g., fatigue, stress, change in weather) can trigger an exacerbation.
Diagnosis and Staging
By the time many patients present for treatment, the diagnosis of COPD is apparent. In addition to symptoms of dyspnea, chronic productive cough, and functional limitations, patients can show physical findings of lung hyperexpansion (increased lung span on percussion, increased thoracic AP diameter, and use of accessory muscles of respiration). Extrathoracic signs include peripheral or central cyanosis, nail clubbing, and signs of increased central venous pressure or even right-sided heart failure. Box 18-3 presents the differential diagnosis and distinguishing features of COPD suggested by the GOLD guidelines. Any patient who develops COPD without a significant smoking history, or any patient developing COPD before age 45, should be screened for α1-antitrypsin deficiency. HRCT can help identify granulomatous or interstitial lung diseases or provide evidence of bronchiectasis.
Box 18-3 Differential Diagnosis of COPD∗
∗ These features tend to be characteristic of the respective diseases but do not occur in every case. For example, a person who has never smoked may develop COPD, especially in the developing world where other risk factors may be more important than cigarette smoking, and asthma may develop in adult and even elderly patients.
(GOLD Guidelines)
From Global Initiative for Chronic Obstructive Lung Disease: Executive Summary: Global Strategy for the Diagnosis, Management, and Prevention of COPD, Updated 2008, p 39. http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=996.