Chapter 144 Prevention of Thrombophlebitis and Pulmonary Embolism in Total Knee Arthroplasty
Knee Surgery and Thrombotic Risk
TKA is one of the most commonly performed orthopedic procedures. Statistics show that 542,000 TKA procedures were performed in 2006 in the United States, with the aim of relieving pain and restoring function and mobility.67
Deep vein thrombosis (DVT) is the most common postoperative complication of TKA and the most frequent cause of hospital readmission. Patients undergoing major orthopedic surgery on the lower limbs, such as TKA or total hip arthroplasty (THA), are at particularly high risk for DVT when compared with other surgical or medical patients, with the incidence of total DVT after TKA ranging from 41% to 85%.36 The incidence of thrombi in the proximal veins, which have been shown to give rise to a higher rate of embolization than thrombi in more distal veins, is thought to range from 5% to 22%.38 Pulmonary embolism (PE), a potentially fatal complication of DVT that occurs when a fragment of a thrombus breaks off and embolizes in the lungs, is less well documented, but published studies have reported rates of 0% to 10% for total PE and 0% to 1.7% for fatal PE.11,36 In addition to being a major cause of death, DVT can be a significant cause of morbidity through recurrent thrombosis or post-thrombotic syndrome, and can result in pain, swelling, and leg ulceration in advanced cases.72
The surgical procedure itself carries with it an intrinsic risk for DVT. Injury to the venous endothelium as a result of operative positioning and manipulation, thermal injury from bone cement, anterior tibial subluxation, and flexion of the knee for an extended time may result in foci of vascular damage that represent an increased risk for thrombosis. The trauma of the procedure itself results in sustained activation of tissue factor and other clotting factors, which then localize at sites of vascular injury and in areas of venous stasis. Postoperative reduction in levels of antithrombin III and inhibition of the endogenous fibrinolytic system may allow continued growth of the thrombus. In addition to the risks associated with surgery itself, the risk for DVT can be compounded by the presence of acquired or congenital risk factors such as advanced age, obesity, cancer, and inherited coagulation disorders.3 Because patients undergoing TKA frequently are of advanced age, have concomitant disease, and may be less mobile for prolonged periods, risk can quickly accumulate.
In addition to the burden of mortality and morbidity associated with DVT and PE, these events impose substantial demands on health care resources.14,69,70 One study showed that the total medical cost for orthopedic surgery patients in whom in-hospital VTE developed was approximately $18,000 higher than in those in whom it did not.71 This finding has been supported by several studies showing that effective thromboprophylaxis in patients undergoing major orthopedic surgery is a cost-effective strategy.42,44,54,65
Options for Thromboprophylaxis in Knee Surgery
Pharmacologic Thromboprophylaxis
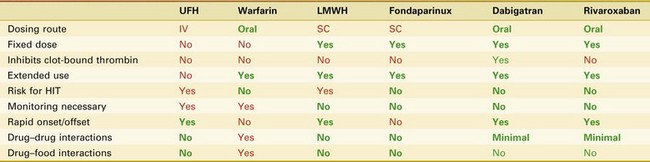
Anticoagulants used for thromboprophylaxis in orthopedic surgery patients act at a variety of sites in the coagulation cascade (Fig. 144-1). Currently available agents include warfarin, LMWHs, fondaparinux, direct thrombin inhibitors such as dabigatran, and direct Factor Xa inhibitors such as rivaroxaban. Unfractionated heparin (UFH) has been widely used for treatment and thromboprophylaxis in medical and surgical patients, but its lower efficacy in TKA patients combined with issues of practicality (need for intravenous administration and laboratory monitoring) has caused it to be used less frequently in this patient population (Table 144-1).19,36
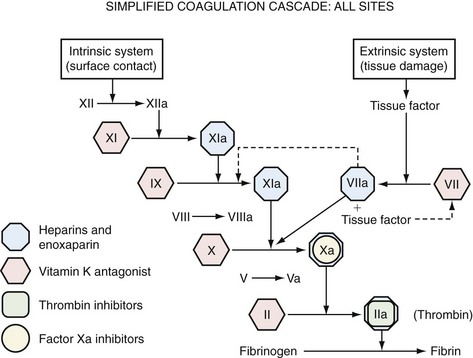
Figure 144-1 Simplified coagulation cascade: sites of action of anticoagulant drugs used in thromboprophylaxis.
Table 144-1 Old and Current Anticoagulants: An Overview of Action and Administration*
HIT, Heparin-induced thrombocytopenia; IV, intravenous; LMWH, low-molecular-weight heparin; SC, subcutaneous; UFH, unfractionated heparin.
* Green is an advantage; red is a disadvantage.
Warfarin
Warfarin has formed the mainstay of anticoagulant therapy for longer than 50 years. In a class of agents known as vitamin K antagonists, warfarin is one of the most widely used drugs for thromboprophylaxis. It acts by inhibiting the cyclical interconversion of vitamin K and its 2,3-epoxide, thus leading to limited carboxylation of vitamin K–dependent clotting Factors II, VII, IX, and X. This action results in inhibition of the extrinsic coagulation pathway (see Fig. 144-1). The anticoagulant effect is delayed for 24 to 36 hours—the time necessary for replacement of normal clotting factors with the decarboxylated form; however, the full anticoagulant effect does not occur until some 72 to 96 hours later, when all vitamin K–dependent factors have been completely replaced.6 Warfarin has been the standard for hip arthroplasty and remains one of the most widely used and effective methods of thromboprophylaxis in THA. Fewer data are available on its safety and efficacy in TKA patients.
The efficacy of dose-adjusted warfarin in patients undergoing TKA has been assessed in a number of randomized clinical trials; analysis of nine of these studies has produced relative risk reductions of approximately 27% in the incidence of pooled DVT when compared with no preventive measures.36,37 The incidence of venographically detected asymptomatic DVT has been shown to be relatively high (25% to 50%) after warfarin therapy; although most such events remain clinically silent, 10% to 20% of calf vein thrombi extend to the proximal leg veins, where thrombosis is associated with a major risk for PE.57 In contrast to asymptomatic DVT, the incidence of symptomatic VTE is low in patients receiving warfarin. In one study, the incidence of symptomatic VTE in 257 TKA patients who received warfarin for 10 days at doses adjusted to achieve an international normalized ratio (INR) of 1.8 to 2.5 was 0.8%.76
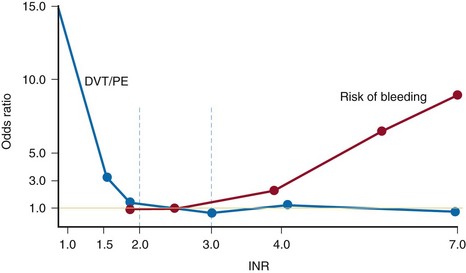
Although warfarin offers the convenience of oral administration, its use in clinical practice can be complicated. It has a narrow therapeutic window (Fig. 144-2) and shows considerable interindividual variability in its dose-response relationship.17 With a narrow therapeutic window, an INR of less than 2.0 will be associated with low risk of bleeding but reduced efficacy and, therefore, increased risk for DVT and PE. If the INR rises above 3.0, the risk for thromboembolic complications is low, but the risk of bleeding, both at the surgical site and remotely, rises exponentially. Furthermore, warfarin is subject to numerous dietary and drug interactions,6 and the need for regular dose adjustments to achieve and maintain a target INR can lead to additional problems such as nonadherence to medication or communication difficulties between physician and patient.6 Therefore, it is very difficult to keep the patient inside that narrow therapeutic window, where both efficacy and safety exist.
Low-Molecular-Weight Heparins
LMWHs are derived from UFH by chemical or enzymatic depolymerization and have a mean molecular weight of 4000 to 5000 Da. Like heparin, LMWHs produce their major anticoagulant effect by activating antithrombin. This interaction increases the inhibitory activity of antithrombin III against procoagulatory serine proteases such as Factors IIa (thrombin), IXa, and Xa (see Fig. 144-1). When compared with UFH, LMWHs offer a more favorable pharmacokinetic profile and a more predictable dose response, plus a reduced risk of heparin-induced thrombocytopenia—a potentially serious complication of heparin therapy that can lead to platelet aggregation and risk for venous and arterial thrombosis.83 Because of reduced anti–Factor IIa activity relative to anti–Factor Xa activity, monitoring of the activated partial prothrombin time is not required, thus allowing subcutaneous administration of a fixed dose without the need for laboratory monitoring. Accordingly, LMWHs are suitable for extended thromboprophylaxis outside the hospital setting,53 because the risk for VTE may persist for several weeks beyond the time of hospital discharge (currently 3 to 4 days postoperatively).30,31
Extensive data have shown that LMWHs are effective and safe in the prevention of VTE after TKA. A study published in 1994 concluded that LMWH was significantly more efficacious than low-dose warfarin in TKA. The incidence of DVT was found to be 26% (6% proximal) in the LMWH group versus 43% (10% proximal) in the warfarin group. Safety was similar between groups, with no increased risk of bleeding.32 For six randomized studies comparing LMWHs with warfarin, the pooled incidence of DVT was found to be 33% and 48%, respectively, and that of proximal DVT was 7.1% and 10.4%, respectively.*
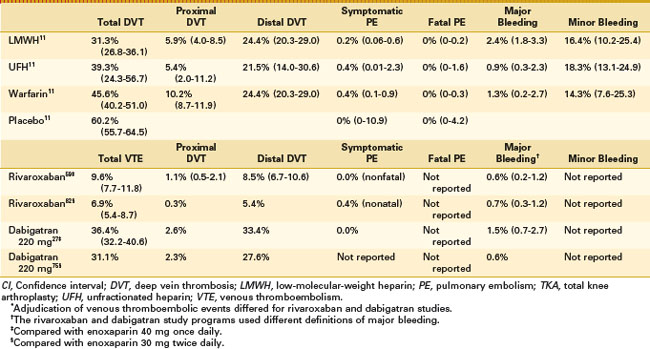
The superior efficacy of LMWH over warfarin has been confirmed in a number of meta-analyses. In one analysis of data from 10 randomized trials involving approximately 3000 patients, the relative risk for DVT in patients receiving LMWH was 0.73 versus treatment with warfarin or dose-adjusted UFH, whereas the relative risks for proximal DVT and PE were 0.58 and 0.55, respectively.49 A further meta-analysis of 14 studies involving a total of 3482 patients found that the total risk for DVT was 31.3% in LMWH-treated patients versus 45.6% in warfarin-treated patients and 60.2% in patients receiving placebo (Table 144-2).11 LMWH produced significantly greater reductions in total (P = .0001), distal (P = .0001), and proximal (P = .0002) DVT when compared with warfarin—an observation that has clinical relevance in light of the fact that proximal DVT is associated with a much higher risk for PE.
Indirect Factor Xa Inhibitors
Fondaparinux is a synthetic analogue of the pentasaccharide sequence involved in binding UFH or LMWH to antithrombin III—the major endogenous inhibitor of Factor Xa and thrombin (see Fig. 144-1). Binding of fondaparinux to antithrombin III leads to increased inactivation of Factor Xa and acts as an indirect Factor Xa inhibitor. The drug has a predictable dose response, a long plasma half-life of approximately 17 hours, and 100% bioavailability, thus permitting once-daily subcutaneous injection without laboratory monitoring.46
The antithrombotic efficacy of fondaparinux has been evaluated in a randomized, double-blind trial involving 1049 patients undergoing elective major knee surgery.8,81 Thromboprophylaxis was administered for 5 to 9 days, starting 6 hours postoperatively. The incidence of documented VTE on day 11 was 12.5% in patients receiving fondaparinux (2.5 mg) versus 27.8% in those receiving the LMWH enoxaparin (30 mg), for a relative risk reduction of 55.2% (P < .001). However, major bleeding was significantly more common with fondaparinux than with enoxaparin (2.1% vs. 0.2%, respectively; P = .006).
Other limitations to the use of fondaparinux in TKA have been identified. The drug should not be used in patients who are older than 75 years, weigh less than 50 kg, or have a creatinine clearance less than 30 mL/min because the risk of bleeding is increased significantly. Because it has a long half-life of 17 to 20 hours, the surgeon must wait 2.5 days after the last dose for the drug to clear sufficiently so that bleeding is not increased if one has to reoperate.7
Direct Factor Xa Inhibitors
Direct Factor Xa inhibitors bind directly to Factor Xa and do not require antithrombin to exert their effects, thus preventing subsequent reactions leading to thrombin generation.85 They inhibit both free and platelet-bound Factor Xa, and Factor Xa bound to the prothrombinase complex.85 The orally active direct Factor Xa inhibitors could improve convenience and encourage patient compliance (particularly in the outpatient setting) compared with the parenteral agents. Apixaban and rivaroxaban are two such agents; currently rivaroxaban has been approved in Canada and several other countries for the prevention of venous thromboembolism in adult patients undergoing elective hip or knee arthroplasty (see Chapter 141 for additional details). In two phase III trials, rivaroxaban demonstrated superiority to enoxaparin 40 mg once daily and to enoxaparin 30 mg twice daily for the prevention of VTE after TKA (see Table 144-2).58,82
Direct Thrombin Inhibitors
Direct thrombin inhibitors, in contrast to indirect thrombin inhibitors, bind to thrombin itself and thus block the interaction of thrombin with its substrate.85 Direct thrombin inhibitors offer a number of potential advantages over indirect inhibitors. Because they do not bind to plasma proteins, they offer a more predictable anticoagulant effect than indirect inhibitors; they also offer the advantage of being effective against both circulating and clot-bound thrombin (whereas heparins are ineffective against clot-bound thrombin, which remains biologically active).85 Their oral route of administration provides added convenience and encourages patient compliance (particularly in the outpatient setting). Dabigatran etexilate is one such agent; it has been approved in Canada and in several other countries for the prevention of venous thromboembolic events in adult patients undergoing elective THA or TKA (see Chapter 141 for additional details). Two phase III studies have compared dabigatran etexilate with enoxaparin for the prevention of VTE after TKA. Dabigatran etexilate demonstrated noninferiority to enoxaparin 40 mg once daily,27 but failed to meet prespecified criteria for noninferiority compared with enoxaparin 30 mg twice daily75 (see Table 144-2).
Aspirin
Aspirin is appealing to the clinician because of its easy administration, minimal bleeding complications, and relative cost-effectiveness. It irreversibly binds and inactivates cyclooxygenase on circulating platelets, which in turn inhibits thromboxane production, a prostacyclin required for platelet aggregation. Aspirin has been shown to be less effective than other prophylactic measures in a number of clinical trials and is not recommended as a sole method of thromboprophylaxis.36 In one randomized clinical trial comparing enoxaparin and aspirin with venographic endpoints, the overall DVT rate after TKA was 79%, with a proximal rate of 33%.39 Other studies have shown DVT rates between 47% and 80%, with most of the clots being distal.
Bleeding Issues
All forms of pharmacologic thromboprophylaxis must be managed properly to minimize the risk of bleeding. In terms of bleeding risk, meta-analyses comparing LMWHs and warfarin have shown that, when compared with warfarin, the use of LMWHs is not associated with increased risk for bleeding complications (see Table 144-2). The meta-analysis performed by Howard and Aaron49 found that LMWHs are more effective than warfarin, UFHs, or placebo in preventing DVT after TKA, but it is important to note that they have been found to be as safe as well. This result is supported in studies by Leclerc and associates60 and Heit and colleagues,45 who reported no differences in major or minor bleeding during or after TKA.
As demonstrated in the study of Levine and coworkers,61 patients not receiving any pharmacologic prophylaxis still have a bleeding rate of 2% to 3%; this has been consistent in the literature throughout the years. Bleeding is multifactorial and, as such, will occur in placebo patients. It is important for the surgeon to minimize the risk for additional bleeding when using pharmacologic agents by giving drugs at proper doses and in accordance with the appropriate dosing schedule.
Mechanical Thromboprophylaxis
IPC devices can play a role in the prevention of DVT, particularly in patients who have contraindications to anticoagulation treatment. IPC devices have been found to be effective in a number of small studies involving TKA patients.10,41,51,64 However, patients must wear the devices for 17 to 20 hours each day, and this is not practical or realistic when they are getting out of bed and bearing full weight on the first postoperative day and being discharged 3 to 4 days postoperatively.
New, mobile pneumatic compression devices have been evaluated. Continuous enhanced circulation therapy (CECT) compression systems have been compared with LMWHs for the prevention of VTE. After THA, CECT was found to be noninferior to enoxaparin (30 mg twice daily) in reducing the incidence of VTE and to have a lower rate of bleeding.18 When combined with LMWH, CECT significantly reduced the incidence of DVT compared with LMWH alone after TKA (6.6% compared with 19.5%, respectively; P = .018)25 and compared with standard compression devices after joint arthroplasty (1.3% vs. 3.6%, respectively).35 Compared with standard compression devices, patients using the CECT system had better compliance and shorter hospital stays.35 CECT systems in combination with low-dose aspirin have also been found (via venography at 5 to 8 days after surgery) to be more effective than enoxaparin for the prevention of DVT after THA or TKA.38
Limited data are available regarding the use of GCSs in TKA patients, but available evidence suggests that GCSs have little effect on risk for VTE in this group.50,61 This may reflect technical issues in TKA, such as the use of a thigh tourniquet, which impairs venous drainage, damages the endothelium, and confines coagulation factors below the tourniquet, thus increasing the risk for thrombosis. In general, GCSs are not considered an adequate means of thromboprophylaxis for TKA patients.36
The use of VFPs is based on the large volume of blood contained in the foot and the beneficial effects of the physiologic foot pump.79 Two small trials have found that VFPs are effective in TKA patients,86,89 whereas others have shown that this approach is less effective than therapy with LMWHs.9,68 VFPs may have value in the early postoperative stage when used in conjunction with pharmacologic thromboprophylaxis, but use of VFP as the sole method of thromboprophylaxis is not recommended.36
As stated, to be most effective, mechanical forms of prophylaxis should be applied during or immediately after surgery and should be worn continuously, at least until the patient is fully ambulatory. Mechanical means of thromboprophylaxis generally require 17 to 21 days of treatment to be effective, yet knee surgery patients typically are discharged 3 days after surgery. This means that the usefulness of mechanical methods can be limited by poor compliance, incorrect use, and inability to continue thromboprophylaxis after hospital discharge. Interest is increasing in the “stacked modality” approach, whereby several types of thromboprophylaxis are used concurrently, such as the combination of IPC or foot pumps with LMWH or warfarin. Results from the Global Orthopaedic Registry (GLORY) suggest that North American surgeons are three times more likely to adopt this approach than surgeons elsewhere.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree