Fig. 16.1
Structure and organelles of platelets
Table 16.1
Mediators found in alpha granules of platelets
Group | Mediators | Functions |
|---|---|---|
Adhesive proteins | VWF, TSP-1, TSP-2, laminin-8 | Hemostasis and coagulation, cell-to-cell contact |
Coagulation factors and inhibitors | Factor V/Va; protein S; protease nexin-1, 2; protein C inhibitor | Thrombin generation and cell proliferation |
Fibrinolytic factors and inhibitors | Plasminogen, PAI-1, u-PA, α2-macroglobulin | Plasmin production and vascular remodeling |
Proteases and inhibitors | MMP-1, -2, -4, -9; TIMPs-1,4; C1 inhibitor, α1-antitrypsin | Angiogenesis and vascular remodeling |
Growth and mitogenic factors | PDGF, IGF-1, EGF, VEGF, bFGF, HGF, BMP-2, CTGF | Chemotaxis, proliferation, differentiation, angiogenesis |
Chemokines and cytokines | TGF-β1, β2, IL-1, RANTES, IL-8, MIP-1α, MIP-2, SDF-1α, PF4, angiopoietin-1, endostatin | Angiogenesis, chemotaxis, vascular remodeling, bone formation, cellular interactions |
Antimicrobial proteins | Thrombocidins, quinocides | Bactericidal and fungicidal effect |
Others | Chondroitin sulfate-4, immunoglobulins G and M, amyloid β-protein precursor, complement factor H | Various effects |
Preparation of PRP
Many protocols are being developed for obtaining PRP, and all methodologies have similar principles:
1.
Anticoagulant is added into blood collection tubes. Citrate-phosphate dextrose, anticoagulated citrate-dextrose A (ACD-A), or trisodium citrate is preferred. ACD-A anticoagulant is also preferred for preserving apheresis platelets. Dextrose is a source for platelets, and phosphate is used as a buffer solution. However, a commonly used anticoagulant, EDTA, is not preferred because EDTA-bound platelets are rapidly removed from plasma and it may harm the platelet surface integrity; therefore, a complete cell viability is essential for growth factor secretion of the platelets [4].
2.
Collected blood is centrifuged. In the first centrifugation, blood is collected into layers (Figs. 16.2 and 16.3). Then, the layers are collected according to the type of PRP preferred (leukocyte-reduced or leukocyte-platelet-rich plasma). For leukocyte-reduced PRP, superficial buffy coat (BC) and plasma above the BC are collected, and a second hard-spin centrifugation is applied. In the final product, upper part of the plasma, called as platelet-poor plasma (PPP) is discarded. For obtaining leukocyte-platelet-rich plasma (L-PRP), BC, superficial red blood cells, and plasma above the BC are collected. After a second hard spin, BC and a few milliliters above the BC are collected with eye-balling. However, in Anitua methodology, one-spin centrifuge is being used, and after a soft spin (460–580 g, 8 min), the lower part of the plasma above the BC is collected and named as plasma rich in growth factors (PRGF) [4, 13–15].
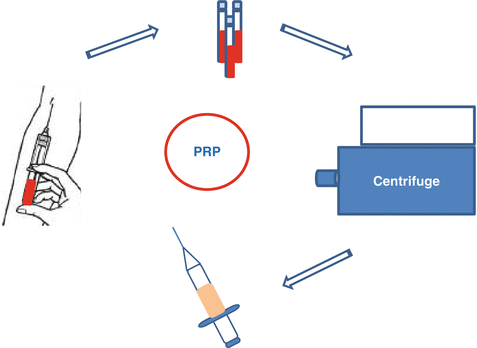


Fig. 16.2
Centrifuge method for PRP preparation

Fig. 16.3
Leukocyte-reduced platelet-rich plasma. After a soft-spin centrifugation, blood is separated into different layers. RBC red blood cells, BC buffy coat, PRP platelet-rich plasma, PPP platelet-poor plasma
3.
PRP can be activated and platelet-rich fibrin (PRF) can be obtained if needed. For activating and aggregating platelets, ADP, thrombin, collagen, arachidonic acid, and calcium chloride can be used in different doses. Platelet degranulation mostly occurs in 10 min and finishes in 1 h. PRF is obtained (Fig. 16.4) [15, 16].


Fig. 16.4
Platelet-rich fibrin (PRF) obtained by adding 10 % calcium into PRP. After 10 min, PRF is ready for use as a source of growth factors
In PRP applications, a gelous cement may be obtained from activated samples and applied to the area of lesion. The PRP method is the in vitro imitation of in vivo coagulation process and application to the area of lesion.
Application and Outcomes
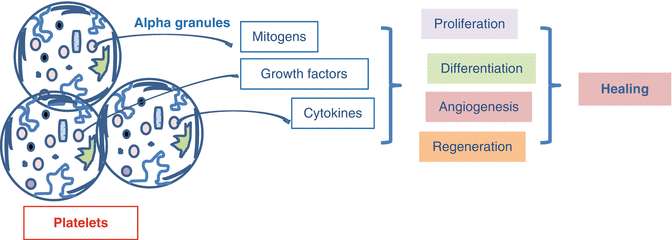
The expected outcome of PRP is its application to the area of lesion as a concentration of growth factors found in alpha granules (Fig. 16.5). With this method, it is possible to apply many growth factors together much more cheaply compared to the cost of a single recombinant growth factor [17]. Additionally, the fibrous network, created by the activated platelets in PRP, is thought to have a contribution in the healing process via formation of regenerative matrix support [18]. PRP enhances the wound healing process and epithelization two to threefold and decreases scar formation via its content of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) [19]. In some animal studies, the growth factors released from PRP were shown to have favorable contributions to the osteoblasts or stromal cells, the vital cells of solid tissue [20]. In an in vitro study, it was stated that PRP favorably affected the development of chondrocytes and showed anabolic effects on chondrocytes, while PRP formulations having high leukocyte count might have an increased catabolic effect [21]. Ultrasonography-aided intratendineal application of PRP contributes to rapid healing of tendons [22]. In addition to these data, it is supposed that the application of autologous PRP remarkably eliminates the risk of cross reactivity, immune reactions, and disease contamination [23].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








