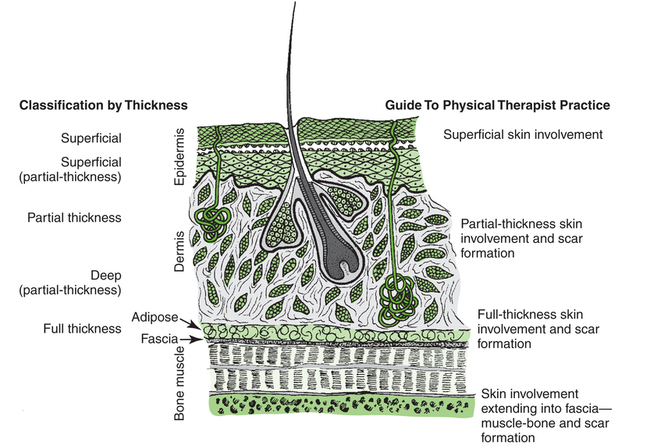
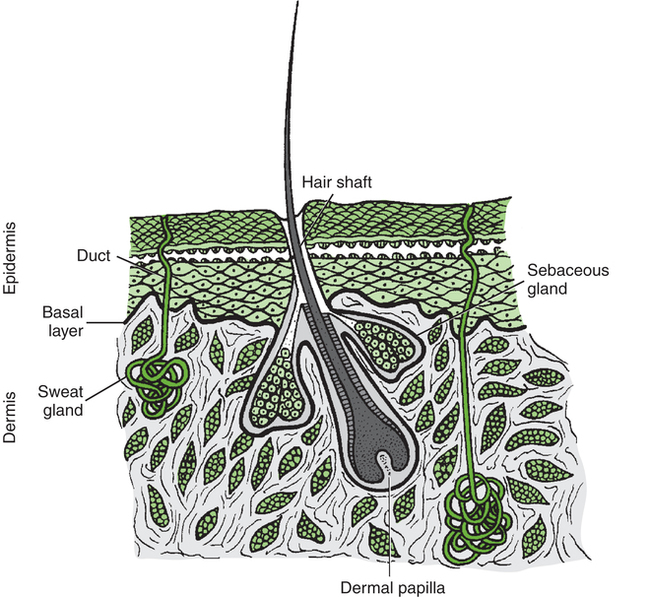
After reading this chapter, the reader will be able to: The integument, the largest organ of the body, ranges from about 1 to 4 mm in thickness and consists of two layers—the epidermis and the dermis. Beneath the dermis lies a layer of subcutaneous tissue. The integument is basically a protective organ, but it also plays a role in temperature control and provides important sensory information regarding the environment. Figure 11-1 illustrates the structure of the skin and its appendages. Wound healing is commonly described in three phases: the inflammatory phase, the proliferative phase, and the remodeling phase.1 Each of the phases, along with applicable interventions for each phase, are discussed briefly in this section. It is important that all the phases of wound repair occur simultaneously to some extent. For example, inflammation can occur while the proliferative process is in progress. With any injury comes an inflammatory phase during which repair of the damaged tissue is initiated.2 Local cellular and vascular reactions are included in this wound-healing phase.3 Initial blood loss is decreased by the immediate vasoconstriction of vessels. The vasoconstrictive response may last about 5 to 10 minutes. This time frame also allows the accumulation of platelets and the formation of temporary “platelet clots” along the damaged endothelial lining of the vessels. Activation of the clotting cascade leading to the eventual formation of fibrin clots begins at this time. The period of vasoconstriction is followed by an episode of vasodilatation and increased capillary permeability. Leukocytes, which are chemotactically recruited to the wound site, are delivered by the increased flow of blood with vasodilatation. Early battles against infection are waged at this point by neutrophils.4–7 Macrophages also migrate to the wound site to phagocytose wound debris and spent cells. Macrophages release factors important in wound repair, such as cytokines, growth factors, and collagenases.8 Lymphocytes also follow neutrophils into the wound site. They play an important role in the immune response because they release factors that stimulate macrophages and fibroblasts.9 The increased capillary permeability during inflammation can lead to the formation of local edema. Edema hinders healing by reducing the local arterial, venous, and lymphatic circulation and increases the chance of infection for the same reasons. Edema may also restrict motion, which increases the possibility of tissue fibrosis. Collagen is the chief protein produced by fibroblasts.10 Collagen fibrils formed by fibroblasts combine and form collagen fibers. Collagen fibers supply the preponderance of strength to the wound. The strength lies in the collagen fiber, not in the amount of collagen at the wound site,11 so a patient does not need a big scar to have a strong and well-healed wound. Ground substance (glycosaminoglycans, water, and salts) occupies the space among the elastin, collagen, vascular structures, and other cells in the healing wound.12 The ground substance allows cell proliferation and migration and provides some cushion for the healing tissue. Angiogenesis (the formation of new blood vessels) begins during the inflammatory phase of healing, but the majority of regrowth occurs during the proliferative healing phase.13 Vascular genesis is important for the distribution of nutrients and oxygen to cells at the site of healing. One other concern associated with the proliferative phase of healing is wound contraction. Wounds begin to contract slightly during inflammation; however, aggressive contraction at the wound commences during the proliferative phase. Fibroblasts, particularly myofibroblasts, have contractile capability.14–16 It appears that the physiologic function of wound contraction is to decrease the surface area of the wound, but contraction takes place in wounds of all sizes. Although potentially beneficial in small wounds, contraction is more frequently the cause of decreased mobility and cosmetic change, particularly in wounds associated with joints. Physical therapy interventions for the proliferative phase of healing may include wound care, edema management, positioning, splinting, cautious passive range-of-motion exercises, active range-of-motion exercises, ambulation, and functional activities such as activities of daily living, similar to interventions during the inflammatory phase. In addition, active assisted range-of-motion exercises, stretching, strengthening exercises, and endurance exercises may be appropriate. During this phase wounds must be handled carefully because a wound will not be as strong as normal skin.17 The maturation phase of healing is also often referred to as the remodeling phase. During the maturation phase, collagen continues to be actively deposited while it is also going through active lysis. The balance between the amount of collagen deposition by fibroblasts and the magnitude of collagen lysis influences the ultimate appearance of the scar (if scar formation occurs). If deposition exceeds lysis, either a hypertrophic scar or a keloid scar forms.18,19 Keloid scars differ from hypertrophic scars in that they extend beyond the original boundaries of the wound, they take longer to mature, and they are not associated with contracture, and there may be some biologic distinction between these types of scars.20–22 The maturation phase of wound healing may last for several months. While the phase is active—that is, while collagen is being produced—the wound continues to contract with varying degrees of vitality. As the phase nears its end, wound contraction tends to diminish. Contraction during this phase is often referred to as scar contraction. If scar contraction leads to either a permanent or a semifixed positional fault at a joint, it is referred to as a scar contracture. Race, family history, depth of the wound, size of the wound, patient age, and location of the wound all appear to be factors affecting scar formation.22–25 The variables of repair and patient response to skin wounds include depth of the damage, location of the injury, size of the wound, healing time, and cause of the disruption. The depth of injury probably has the greatest impact on repair and eventual healing of a wound. For example, superficial wounds that leave a majority of the epidermal basal cells intact often heal without complication. Deeper skin damage that destroys much if not all the epidermal basal cell layer may take weeks to heal or require surgical intervention to hasten repair. Generally, the deeper the wound, the longer it takes to heal. Figure 11-2 illustrates depths of wounds and the integumentary structures involved at the varying depths. Wounds caused by arterial insufficiency are most commonly situated on the foot or ankle, but they also occur at other locations. These wounds are caused by primary loss of vascular flow to an anatomic site, which leads to tissue death.26 Venous insufficiency (venous stasis) can also lead to ulceration of the skin and generally occurs on the lower part of the legs.27 Venous stasis may result from venous hypertension, venous thrombosis, varicose (dilated) veins, or obstruction of a portion of the venous system. The precise cause of ulcers caused by venous stasis has not been determined. Various theories forwarded to explain venous stasis ulceration include the notion of “fibrin cuff formation” that occurs as a result of an increase in capillary leakage of fibrinogen (as well as other large molecules) secondary to venous hypertension.4 Fibrin then accumulates in the interstitial space and around capillaries and produces an obstacle to the transportation of oxygen and nutrients to tissue. Another theory regarding venous stasis ulcers is referred to as “white cell trapping.” Venous hypertension decreases capillary flow and the subsequent removal of leukocytes. The trapped cells then occlude capillaries, which leads to ischemic damage and may also release substances that bring about direct local tissue damage. 28–31 Pressure on tissue causes ischemia, producing damage, tissue hypoxia and death, and a wound referred to as a pressure ulcer.32 Only a few hours of pressure can cause severe tissue injury.33,34 Pressure occurs most commonly over areas of bony prominence, such as the sacral or coccygeal area, ischial tuberosity, heel, lateral malleolus, and greater trochanter. Pressure may increase or decrease, depending on the patient’s position.35 Table 11-1 lists sites at risk for pressure ulcers by position. For example, simply being positioned incorrectly in bed can damage the skin. Table 11-1 Body Areas Commonly at Risk for Pressure Ulcer Development Data from Kosiak M: Etiology and pathology of ischemic ulcers, Arch Phys Med Rehabil 62:492-498, 1981.
Physical Therapy for Integumentary Conditions
 Discuss the structure and function of the skin
Discuss the structure and function of the skin
 Discuss the process of wound healing, including the three major phases—inflammation, proliferation, and maturation
Discuss the process of wound healing, including the three major phases—inflammation, proliferation, and maturation
 Describe common problems associated with the integument (including vascular compromise, trauma, and disease) and the basic examination principles related to those conditions
Describe common problems associated with the integument (including vascular compromise, trauma, and disease) and the basic examination principles related to those conditions
 Describe basic intervention principles and strategies necessary in complete patient care (including prevention, management, and education)
Describe basic intervention principles and strategies necessary in complete patient care (including prevention, management, and education)
General Description
Integument
Wound Healing
Inflammatory Phase
Proliferative Phase
Maturation Phase
Additional Considerations
Common Conditions
Vascular Compromise
Position
Areas at Risk
Supine
Occiput, elbows, scapulae, spinous processes, sacrum, coccyx, heels
Seated
Elbows, spinous processes, sacrum, coccyx, ischial tuberosities, greater trochanters, heels
Side-lying
Ear, shoulder, elbow, greater trochanters, medial and lateral aspects of knees, medial and lateral malleoli, heels
Prone
Forehead, nose, chin, anterior of shoulder, iliac crest, patella, dorsal surface of foot or toes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Physical Therapy for Integumentary Conditions