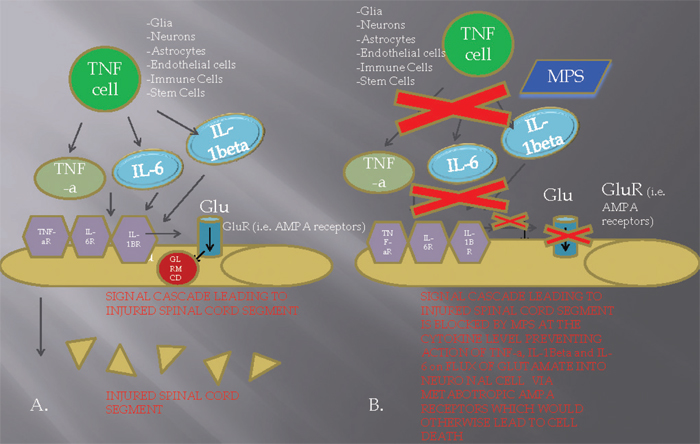
10 Key Points 1. High-dose steroids are commonly used in the treatment of acute spinal cord injury. 2. Significant limitations exist in both the animal and the clinical literature supporting the use of steroids in acute spinal cord injury. 3. Potential complications exist that are associated with high-dose steroids in patients with acute spinal cord injury. 4. Given the risks and benefits, high-dose steroids should be used cautiously and with careful observation in patients with acute spinal cord injury. Advances in the understanding of basic physiology and neurobiology in spinal cord injury (SCI) and the development of therapeutic strategies have progressed at a frustratingly slow rate. This is in spite of the estimated incidence of traumatic SCI in the United States of 15 to 40 new cases per million population, or 12,000 cases annually.1–3 Although the figures for incidence are high due to the impact on the younger population, the estimates of prevalence vary from ∼ 183,000 to 230,000 cases in the United States, or the equivalent of 700 to 900 cases per million. The direct cost of SCI is estimated to be in excess of $7 billion annually1,2 and bears the greater cost of loss of life, productivity, emotional suffering, and diminished quality of life for patients and families.1–3 During the last 2 decades, new hope emerged for patients with SCI in the form of several promising therapies heading toward clinical trial. These proposed therapies included tirilizad mesylate, GM-1 ganglioside, thryotropin-releasing hormone (TRH), gacyclidine, naloxone, and nimodipine. Unfortunately, these therapies were unable to demonstrate efficacy for neurological improvement and thus were not accepted by the medical community.4–6 Acute SCI is believed to involve a two-step mechanism.2 The primary mechanism involves the initial, traumatic mechanical injury due to local deformation and energy transformation directly on the spinal cord parenchymal tissue. The secondary mechanism encompasses a cascade of biochemical and cellular processes that are initiated by the primary process, often leading to further cellular damage and potentiating cell death (Fig. 10.1).2 The secondary mechanism of acute SCI was first postulated by Allen7 in 1911 after he described an improvement in neurological function with the removal of posttraumatic hematomyelia in dogs after a traumatically induced acute SCI. The effects of free radicals, as advocated by Demopoulos et al.,8 were thought to be crucial to the injury process. The focus of research has shifted to the role of calcium, opiate receptors, and lipid peroxidation. Recently, research has implicated apoptosis, intracellular protein synthesis inhibition, and glutaminergic mechanisms, among a myriad of pathophysiological pathways that mediate secondary injury mechanisms (Fig. 10.1). There is considerable evidence that the primary mechanical injury initiates a plethora of secondary injury mechanisms, including the following: (1) vascular changes, including ischemia, impaired autoregulation, and microcirculatory derangements9; (2) ionic derangements10; (3) neurotransmitter accumulation, including serotonin or catecholamines and extracellular glutamate, the latter causing excitotoxic cell injury (Fig. 10.1)11; (4) arachidonic acid release and free radical production11 and lipid peroxidation12; (5) endogenous opioids; (6) inflammation; (7) loss of adenosine triphosphate–dependent cellular processes; and (8) programmed cell death or apoptosis.2 Methylprednisolone has been proposed to act by reducing secondary injury, in part by the scavenging of lipid peroxyl radicals.13 Consequently, MPSS is thought to inhibit the lipid peroxidation (LP) cascade and, hence, preserve neurons, axons, myelin, and intracellular organelles, including the mitochondria and nucleus, by preventing free radical injury. However, more recent studies have suggested that methylprednisolone acts preferentially on glial cells with a diminished effect on neurons. MPSS has also been shown to inhibit oligodendrocyte death via activation of the glucocorticoid receptor (GR) (Fig. 10.1), which binds to the STAT5 receptor resulting in the upregulation of the expression of the blc-Xl gene.13 In monocytes, macrophages, and T lymphocytes, the response to the activation of the GR is opposite, resulting in apoptosis. It is postulated that the antiapoptotic effects are due to the increased expression of the neuroprotective cytokine erythropoietin, which is seen in oligodendrocytes. Increasing knowledge of the posttraumatic LP mechanism in the 1970s and early 1980s prompted the search for a neuroprotective pharmacological strategy aimed at antagonizing oxygen radical-induced LP in a safe and effective manner. Attention was focused on the possibility that glucocorticoid steroids might be effective inhibitors based upon their high lipid solubility as well as their ability to intercalate into artificial membranes between the hydrophobic polyunsaturated fatty acids of the membrane phospholipids, thereby limiting the propagation of LP chain reactions.13 Fig. 10.1 Mechanistic diagram describing the potential action of methylprednisolone (MPS) on cytokine [tumor necrosis factor-α, (TNF- α), interleukin (IL)-6, IL-1β] formation and release by TNF-producing cells (i.e., glia, neurons, astrocytes, endothelial cells, immune cells) in the extracellular milieu of the injured spinal cord tissue. (A) In the acute spinal cord injury setting, release of TNF-α, IL-6, IL-1β by a TNF cell leads to binding of these cytokines to each of their respective receptors (TNF-αR, IL-6R, IL-1βR) in the neuronal cell membrane. Subsequently, a signal is sent for opening of the metabotropic glutamate receptor (GluR) (i.e., AMPA receptor) leading to an extracellular to intracellular flux of glutamate (Glu) into the cell, which facilitates glutamate receptor–mediated cell death (GLRMCD). (B) Administration of MPS in the first 8 hours of injury (preferably during the first 4 hours) leads to an inhibition of cytokine formation in the extracellular milieu, consequently blocking the process of GLRMCD. Hall and Braughler14 investigated the effects of high-dose MPSS (15 to 90 mg/kg IV) on spinal cord electrophysiology. In an initial set of experiments in cats, it was observed that the administration of an intravenous bolus of MPSS could indeed inhibit posttraumatic LP in spinal cord tissue, but that the doses required for this effect were much higher (30 mg/kg) than previously hypothesized or than those empirically employed in the clinical treatment of acute central nervous system (CNS) injury. Further experimental studies, also conducted in cat SCI models, showed that the 30 mg/kg dose of MPSS not only prevented LP but, in parallel, also inhibited posttraumatic spinal cord ischemia. Braughler and Hall14 found that a 30 mg/kg dose of MPSS followed at 2 hours by a 15 mg/kg dose provides significantly better protection against injury-induced ischemia and Ca2+- dependent neurofilament degradation than a single 30 mg/kg dose. With many of these therapeutic parameters (LP, secondary ischemia, aerobic energy metabolism), the dose–response for MPSS followed a sharp U-shaped pattern. The neuro- and vasoprotective effect is partial with a dose of 15 mg/kg, optimal at 30 mg/kg, and diminishes at higher doses (60 mg/kg). The antioxidant neuroprotective action of MPSS is closely linked to the drug’s tissue pharmacokinetics.14 For instance, when MPSS tissue levels are at their peak following administration of a 30 mg/kg intravenous dose, lactate levels in the injured cord are inhibited. The subsequent administration of a second dose (15 mg/kg IV), at the time at which the levels after the first dose have declined by 50%, acts to maintain the peak suppression of lactate and more effectively maintains adenosine triphosphate (ATP) generation and energy charge.15,16 These findings suggested that prolonged MPSS therapy might better suppress the secondary injury process and lead to better outcomes compared with the effects of a single large intravenous dose. Indeed, subsequent experiments in a cat spinal injury model demonstrated that animals treated with MPSS using a 48-hour antioxidant dosing regimen had improved recovery of motor function over a 4-week period.13 Glucocorticoids have been repeatedly shown to inhibit axonal sprouting and synaptogenesis in various CNS regions.17,18 Although it is not known whether these actions occur concomitantly with the acute antioxidant neuroprotective effects, the potential of steroids to attenuate posttraumatic plasticity mechanisms is a serious concern. Thus the potential for steroid side effects, inhibition of plasticity mechanisms, and even neurotoxic actions underscores the fact that glucocorticoids like MPSS are a far-from-ideal approach to dealing with the posttraumatic oxidative stress and LP-related damage and consequent need for antioxidant dosing that continues beyond the first 24 to 48 hours. Despite concerns about the potential inhibitory and neurotoxic effects of MPSS, it is encouraging that high doses of MPSS have actually been reported to diminish axonal dieback of vestibulospinal fibers and to promote their terminal sprouting in transected rat spinal cords.19 Thus the question of whether high-dose MPSS is neuroprotective or neurotoxic may depend on dose selection, timing, and duration of administration and the particular neuronal population in question. Three landmark National Acute Spinal Cord Injury studies examined the use of MPSS for acute traumatic SCI. The first NASCIS study, published in 1984 and 1985,20 compared a high-dose with a low-dose cohort of methylprednisolone (level of evidence: III). The investigated groups included a high-dose methylprednisolone treatment (1000 mg bolus/d) versus the standard dose (100 mg bolus/d) in 330 patients for 10 days after SCI. A placebo was judged unethical because of an assumed benefit from steroid administration and was therefore excluded.21 Motor scores were determined based on the Bracken scale, from the examination of seven muscle groups on each side of the body scored on a 6-point scale. The authors reported the motor and sensory scores from the right side of the body only. No difference in neurological recovery of motor function or pinprick and light touch sensation was observed between the two treatment groups at 6 weeks and at 6 months after injury (p = 0.63 at 6 weeks, and p = 0.59 at 6 months). The lack of a treatment effect was independent of the severity of the initial lesion or the time from injury to starting treatment. To the contrary, significant complications were reported in both treatments, more often in the high-dose MPSS group. Early mortality was statistically greater in the high-dose protocol (relative risk of 3.1 and 1.9, ≤ 14 and 15 to 28 days after injury, respectively). Wound infections were also more prevalent in the high-dose regimen (relative risk of 3.6). Subsequent animal studies completed after NASCIS I suggested that higher doses were required for neuroprotection than were included in the NASCIS I study,15,22 influencing development of the NASCIS II trial.23 The NASCIS II study was designed as a prospective, randomized, controlled clinical trial (level of evidence: II). The study examined intravenous high-dose MPSS (30 mg/kg bolus with 5.4 mg/kg/h for 23 hours); the opioid antagonist naloxone, a proposed neuroprotective drug; and placebo. Intervention was initiated within 24 hours of injury. In the initial analysis of all patients randomized within 24 hours, there was no neurological benefit in either the MPSS-treated group or the naloxone group compared with placebo at 1 year follow-up of 487 enrolled patients. Though not reaching statistical significance, greater complication rates, including wound infection and pulmonary embolism, were found in the steroid group compared with both naloxone and placebo. A post hoc analysis was performed and demonstrated significant differences in patients receiving steroids within 8 hours of injury. Motor and sensory improvements were reported at 6 months, though only motor improvements were found at 1-year follow-up. No benefits were found for administering MPSS in acute nonpenetrating SCI more than 8 hours after injury. Though vigorously defended, no evidence has been presented to support use of the 8-hour stratification, making it an otherwise arbitrary time point found to be significant in the post hoc analysis. Additionally, the calculated motor score differences (16.0 vs 11.2) do not translate into functionally significant improvements. One of the major critiques of this study is that all primary outcome measures of NASCIS II were negative. It is interesting to see that no table or graph exists where data are displayed as a function of time and subjected to mathematical algorithms to establish a correlation. With the multiple post hoc comparisons required to discover these differences, it remains quite possible that the observations reflect random chance alone.21 The NASCIS II has also been widely criticized for its failure to include outcomes important to the patient. This was criticized sharply by Hurlbert,21 raising questions concerning the clinical importance of reported neurological recovery among patients treated within 8 hours. To correct this oversight, the NASCIS III protocol included the American Spinal Injury Association (ASIA) Functional Independence Measure (FIM) assessment. Furthermore, Bracken and Holford24 also published a retrospective estimate of functional recovery in NASCIS II from results modeled in the NASCIS III study. In that report, the authors claimed that the MPSS therapy-related motor function recovery observed in NASCIS II predicted a clinically important recovery in the FIM.24 However, there was a lack of robustness in statistical modeling, limiting the value of this retrospective evaluation. The NASCIS III trial compared the 24-hour infusion of MPSS used in NASCIS II to a 48-hour high-dose MPSS infusion as well as a treatment group that received tirilizad mesylate, a 21-aminosteroid and antioxidant without the negative perceived glucocorticoid effects.23–25 All patients had their treatment initiated within 8 hours of SCI. No placebo group was included because it was again deemed ethically inappropriate to withhold at least the initial large bolus of MPSS. This trial demonstrated no sustained benefit to high-dose MPSS with respect to motor and sensory scores among the 499 randomized patients at 1-year follow-up. Again, a post hoc analysis noted that patients receiving the MPSS bolus between 3 to 8 hours after injury demonstrated improved neurological function at 6 weeks and 6 months, but not at 1 year, when they were given MPSS for 48 hours rather than 24 hours. Patients treated within 3 hours after SCI demonstrated no differences between 24- and 48-hour infusions. Bracken et al., as a result of NASCIS III, recommended that, within 3 hours of injury, patients receive a 24-hour infusion, whereas for those treated between 3 and 8 hours postinjury, a 48-hour MPSS regimen was better than the 24-hour regimen of the NASCIS II protocol.25 Due to the post hoc analysis in the NASCIS II trial, attention was placed on the particular issue of drug delivery within 8 hours and MPSS physiology. Hurlbert21 demonstrated that a physiological rationale does not exist for the selection of 8 hours as a determinant of steroid efficacy. This author stated that the 8-hour window is strictly arbitrary and represents a post hoc finding that is inherently flawed, making this time window questionable. The NASCIS II and III studies reported potential complications due to high-dose steroid administration that may have serious negative ramifications for patients. In the NASCIS II trial there was a 1.5-fold higher incidence of gastrointestinal hemorrhage, twofold higher incidence of wound infection, and threefold higher incidence of pulmonary embolism in the MP group as compared with controls. Similarly, in NASCIS III, the 48-hour regimen was associated with a twofold higher rate of severe pneumonia, a fourfold higher rate of severe sepsis, and a sixfold higher incidence of death than in the 24-hour group.21 Although the differences did not reach statistical significance, the trend is concerning because the impact of these complications can be catastrophic. Further, it is important to note that a priori sample size calculations, based on NASCIS II data, indicate over 1400 patients (sample size for binomial proportion, b = 0.8) would be required to prove statistically that no differences in the rate of wound infection exist between both groups.21 Of more concern is the observation in NASCIS III of a sixfold higher incidence of death due to respiratory complications in the 48-hour MP group than in the 24-hour MP group (p = 0.056), suggesting a higher mortality rate associated with a 48-hour protocol.21 However, the potential for complications associated with 48-hour high-dose MPSS must be balanced against the results of NASCIS III wherein the extension of dosing to 48 hours produces a significantly better neurological outcome compared with the 24-hour regimen in patients treated after 3 hours. The foregoing NASCIS III morbidities are akin to the worrisome complications discovered in the recent CRASH trial, which studied steroids in head injury. In this study, head-injured patients with a Glasgow Coma Scale score of 14 or less received either a loading dose of 2 g of methylprednisolone followed by a 0.4 g/h infusion for 48 hours or matching placebo within 8 hours of injury. This randomized, controlled trial was stopped early when it was discovered at interim analysis that steroid-treated patients had significantly higher all-cause 2-week mortality (21.1% vs 17.9%, p = 0.0001).26 Subsequent follow-up demonstrated that 6-month mortality was also higher in steroid-treated patients (25.7% vs 22.3%, p = 0.0001.28 Interestingly, complications, such as seizures, gastrointestinal bleeding, and infection, were similar in both groups. The authors noted that they were unsure of the mechanism of increased mortality with steroids. The lack of an identifiable etiology, however, does not diminish the validity or importance of the results.26 At the present time, none of the data from the three NASCIS studies support the use of methylprednisolone for penetrating trauma. These authors concluded that patients who sustain an SCI secondary to a gunshot wound or other penetrating injury to the spine should not be treated with steroids until the efficacy of such treatment is proven in a controlled study.27 The utility of high-dose methylprednisolone infusion for acute SCI has not been clearly defined in the medical literature. Methylprednisolone is presently being used off label in the United States for SCI because there is no US Food and Drug Administration (FDA)-approved indication for this use.28 Despite the fact that subsequent clinical studies and critical reviews have challenged the validity of the recommendations that followed the NASCIS studies, failure to administer steroids in acute SCI has been cited in litigation against physicians due to the potential “neuroprotective effect.”28 There is certainly an inherent conflict for clinicians when questioning the potential therapeutic benefit versus complications, given the medicolegal ramifications. Randomized trials of MPSS for the treatment of acute SCI have shown that significant improvements in motor function recovery may exist after treatment with the high-dose regimen within 8 hours of injury. However, this improvement effect appears modest, if present at all, and does not appear to result in significant functional clinical recovery. Nonetheless, even small changes in motor recovery, typically assessed in the MPSS trials on one side of the body, do have the potential to be amplified into meaningful improvements in quality of life.29 The NASCIS II trial results did provide some evidence for the safety of high-dose MPSS.23 However, this is counteracted by evidence in NASCIS III of a rise in mortality due to pneumonia, respiratory distress syndrome, or respiratory failure (grouped together) in the 48-hour methylprednisolone group.24,25 This result was based on six deaths in the 48-hour group and one in the 24-hour group (RR = 6.0; 95% CI 0.73 to 49.3). At present, high-dose MP therapy, although not FDA approved in the United States for acute SCI treatment, continues to be an option for initial medical treatment. Evidence-based medicine has evaluated the potential harmful effects of MPSS versus any modest neurological benefits. Bracken29 has addressed various criticisms and misunderstandings in the form of a recent meta-analysis of the NASCIS and non-NASCIS trials of MPSS in acute SCI. The conclusion was that “high-dose MPSS given within 8 hrs of acute SCI is a safe and modestly effective therapy that may result in important clinical recovery for some patients, although further trials are needed to identify superior pharmacological therapies and to test drugs that may sequentially influence the post-in-jury cascade.”29 In 2001, the Spine Focus Panel30,31 reported that, although methylprednisolone is only modestly neuroprotective, it is clearly indicated in acute SCI because of its favorable risk/benefit profile and the lack of alternative therapies. However, a significant minority of respondents were of the opinion that the evidence supporting the use of steroids in SCI was weak and did not justify the use of this medication. The Spine Focus Panel agreed that, given the devastating impact of SCI and the modest efficacy of MP, clinical trials of other therapeutic interventions are urgently needed. Although it is clear that substantial criticisms have emerged and are valid in regard to the administration of MPSS, the lack of available alternatives makes one reconsider whether to eliminate MPSS from the list of available options. It can be concluded, as a Class II recommendation, that in nonpenetrating trauma, MPSS used within 3 hours of injury at a 30 mg/kg loading dose followed by 5.4 mg/kg/h is not an unreasonable selection for SCI.31 In scenarios where between 3 and 8 hours have elapsed, MPSS used at the dose given earlier for 48 hours becomes a viable option only after the family and the patient are aware of the complications that can occur with long-term administration. Any use of the drug for nonpenetrating trauma more than 8 hours after injury should be avoided. Nevertheless, other groups, such as the AANS/CNS, have been less hopeful on the potential of MPSS to become a standard treatment even in specific settings. Guidelines were published in 2002 by this joint section emphasizing that the available medical evidence does not support a significant clinical benefit from the administration of MPSS in the treatment of patients after SCI for a duration of either 24 or 48 hours.32 They state that “the neurological recovery benefit of methylprednisolone when administered within 8 hours of ASCI has been suggested but not convincingly proven.”32 Furthermore, the accepted position on methylprednisolone in the treatment of acute human SCI is that it should be prescribed knowing that the evidence suggesting harm is more consistent than the evidence for the benefit.32 Another aspect of this controversy that may have dispelled many of these conflicts is based on the fact that MPSS has not gone through the FDA approval process. It is necessary to ask why it has not been presented to the FDA. If the evidence is not strong enough to be presented to the FDA, then is it strong enough that methylprednisolone should be the treatment of choice for acute SCI?28 At the least, the experience with high-dose MPSS can shape the future of SCI research. Hopefully, we, as a community of scientists and physicians, have learned enough from this 20-year-old debate to perform better research so that as new compounds are developed, we can subject them to studies that have fewer methodological flaws. Pearls
Pharmacotherapy in Acute Spinal Cord Injury: Focus on Steroids
 Incidence of Acute Spinal Cord Injury
Incidence of Acute Spinal Cord Injury
 Historical Pharmacological Treatment
Historical Pharmacological Treatment
 Basic Science
Basic Science
Pharmacology of Corticosteroids
Dosing Strategies
Animal Spinal Cord Injury Data
 Clinical Studies
Clinical Studies
Randomized, Controlled Trials
NASCIS I
NASCIS II
NASCIS III
Timing of Administration
Complications with the Use of MPSS
CRASH Study
Penetrating Trauma Indications
 Medicolegal Aspects of Corticosteroid Use
Medicolegal Aspects of Corticosteroid Use
 Critical Appraisal of Data—Evidence-Based Medicine
Critical Appraisal of Data—Evidence-Based Medicine
 Conclusion
Conclusion
 There remains little clinical evidence for high-dose steroids in acute SCI.
There remains little clinical evidence for high-dose steroids in acute SCI.
 Judicious use of high-dose steroids in acute SCI can reduce associated complications.
Judicious use of high-dose steroids in acute SCI can reduce associated complications.
 Patients and families should be educated as to the evidence-based risks and benefits of high-dose steroids in acute SCI.
Patients and families should be educated as to the evidence-based risks and benefits of high-dose steroids in acute SCI.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
