Periprosthetic Fracture
Prevention/Diagnosis/Treatment
Christopher R. Gooding, Donald S. Garbuz, Bassam A. Masri and Clive P. Duncan
Key Points
Introduction
Since the mid-1960s, periprosthetic fractures have posed a difficult surgical problem for orthopedic surgeons.1,2 Treatment is based on whether the fracture occurred during or after the operation, and whether it involves the acetabulum, the femur, or both.
On the acetabular side, fractures can occur intraoperatively in the course of bone preparation or component insertion (particularly when a press-fit technique is used for a cementless acetabular component), or in the revision situation while implant removal is attempted.3 Fractures can also occur postoperatively secondary to traumatic injury, or more commonly in association with a loose component that leads to bone loss and a chronic periprosthetic fracture of the acetabulum, where the superior and inferior halves of the hemipelvis are no longer in continuity.4 The latter, termed pelvic dissociation or discontinuity, requires careful radiographic and intraoperative evaluation so as to avoid a missed diagnosis because standard revision techniques may not be adequate for reconstruction.
Similarly, on the femoral side, fractures can occur during5–7 or after surgery.6,8-14 Missed intraoperative fractures and those treated inappropriately carry a high risk of a poor outcome because of nonunion or malunion. Avoidance of such complications is desirable, and prevention certainly plays a role. It is helpful to classify these fractures to enable the use of a logical treatment algorithm that is based on the stability of the implant (e.g., well fixed or loose) and the bone stock available for reconstruction if a revision procedure is required.
Epidemiology and Risk Factors
The incidence of intraoperative acetabular periprosthetic fracture during cemented total hip arthroplasty (THA) has been reported to be as low as 0.2%.15 However, with the advent of the cementless acetabular fixation, the incidence has increased.16–20 A consistent factor in the development of acetabular fractures is under-reaming of the acetabulum in an attempt to gain initial press-fit stability with a cementless component.3 It has been suggested that under-reaming by as much as 4 mm is acceptable21; however, most now agree that 2 mm or less is safer.22
Postoperative acetabular fractures are relatively uncommon. Berry and associates reported 0.9% prevalence associated with pelvic discontinuity, which was observed at revision.4 Risk factors for early postoperative fracture include early weight bearing on pathologic bone, as reported by Sanchez-Sotelo and colleagues.23
Another group of patients at risk of developing an early postoperative acetabular fracture includes those who have undergone revision of an acetabular socket to a hemispherical or elliptical, uncemented, trabecular metal shell. Springer and coworkers24 reported on seven patients who developed a transverse acetabular fracture at a mean postoperative time of 8 months. The authors concluded that the fractures probably occurred as the result of further weakening of the acetabular bone stock caused by the reaming needed to obtain a good press-fit of a large-diameter shell. Once this weakened bone was subjected to weight-bearing, it would fracture. They recommended (1) protecting the columnar support as much as possible during reaming of the socket, and (2) in high-risk patients, limiting early postoperative weight bearing.
The risk of intraoperative femoral fracture appears dependent in part on the method of fixation used. An incidence of 0.1% to 1% has been reported with cemented stems.25 Berry reported a similar incidence of 0.3% in 20,859 primary cemented stems but 5.4% in 3121 uncemented THAs. This higher incidence in uncemented THAs could be explained by the force sometimes required to achieve primary stem stability.
Revision surgery is associated with an even greater incidence of periprosthetic fracture. Berry reported intraoperative fracture rates of 3.6% of 4813 cemented and 20.9% of 1536 uncemented revision total hip arthroplasties.8 Meek and associates26 described a total of 211 patients who underwent revision with a diaphyseal-fitting cementless stem. Sixty-four (30%) patients sustained an intraoperative fracture.
Fredin and colleagues27 reported 11 (0.56%) periprosthetic fractures following primary total hip arthroplasty in 1961 patients over a 14-year period. Kavanagh25 suggested that the incidence was closer to 1% after primary and 4.2% after revision hip replacement. Lowenhielm and coworkers13 reported a similar incidence of 22 (1.5%) fractures out of a total of 1442 total hip replacements, 14 of which were femoral. They also related that the accumulated postoperative risk of femoral fracture over a 15-year period was 25.3 per 1000.
Beals and Tower28 reviewed 102 revision procedures following 93 periprosthetic fractures. They estimated an incidence of periprosthetic fracture of 1% over the lifetime of the implant. A similar incidence of 1.1% was reported by the Mayo Clinic Joint Registry of postoperative periprosthetic femoral fractures from their large series of almost 24,000 primary THAs.8 A higher incidence of 4% was associated with revision hip surgery, after 6349 revision THAs. Table 102-1 summarizes the published incidence/prevalence of periprosthetic hip fracture.
Table 102-1
Summary of the Published Incidence/Prevalence of Periprosthetic Fractures Associated With Total Hip Arthroplasty (THA)
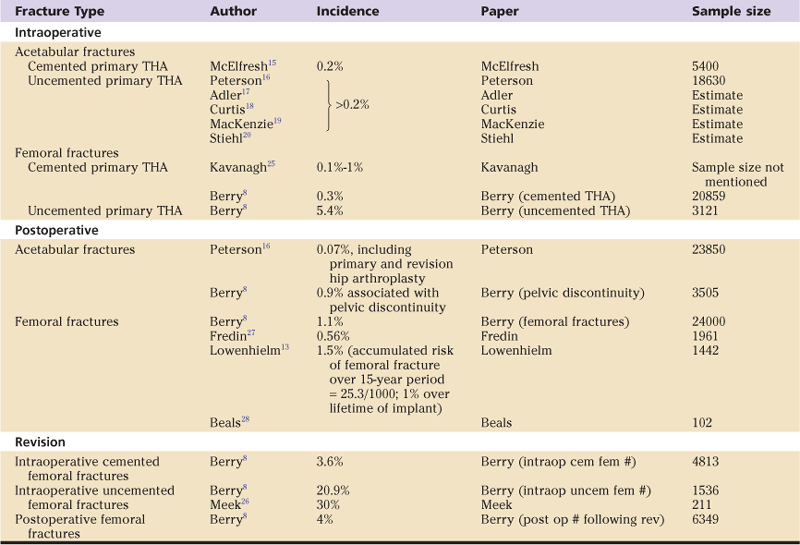
Many reports in the literature indicate that the incidence of late postoperative periprosthetic femoral fractures is increasing.8,25,29-34 This is to be expected, given the advancing age of our population of individuals who have had a THA, and the fact that the risk of periprosthetic fracture is influenced substantially by the age of the patient at the time of hip replacement.35 The risk is low for an individual of normal life expectancy who undergoes primary implantation before reaching the age of 70 years. However, patients who are older than 70 years have a 2.9 times greater risk, and those older than 80 years have a 4.4 times greater risk. This increased risk with advancing age is undoubtedly multifactorial.36
In their review of the Swedish Registry, Lindahl and associates found that higher risk of periprosthetic fracture was associated with patient age and with every year of aging after the primary procedure.36 In this study, the risk ratio for fracture was 1.01 per additional year of aging. Investigators also found that the time from the primary hip arthroplasty was a risk factor, perhaps because of osteolysis, as well as implant loosening. This was confirmed in a later study, when it was demonstrated that the younger the patient is at the time of the primary arthroplasty, the greater is the risk for subsequent fracture,37 although the type of fracture is not influenced by age, gender, or implant type.38
Low-energy falls are responsible for a large number of periprosthetic fractures.39,40 Relatively minor trauma in some studies has accounted for nearly 75% of all periprosthetic fractures.39 Undoubtedly, a multifactorial element is involved as well, and poor bone stock following numerous revisions has been shown to play a significant role.39 Falls commonly occur in the home (66%); only a small number occur outdoors (18%).28
Female gender has long been thought of as a risk factor for periprosthetic fracture.5,10,28,34 However, the Finnish Joint Registry showed no difference in fracture rate between sexes.40 Any perceived risk associated with gender is undoubtedly affected by a number of potential confounding factors, so it is difficult to conclude that gender alone is responsible for an increased fracture rate.
Less controversial is that osteoporosis is a risk factor for periprosthetic femoral fractures post THA.36,41-43 Beals and associates28 observed that 38% of patients in their study had previously sustained a fragility fracture such as a vertebral fracture, and many of their patients had osteopenia. Wu and colleagues, using Singh’s index for osteoporosis, found that preoperative osteoporosis was a significant predictor of fracture.35 A large number of periprosthetic fractures occur secondary to low-energy falls, again suggesting that bone fragility plays a role.36
Other comorbidities before hip arthroplasty may be associated with the risk of periprosthetic fracture. Rheumatoid arthritis can result in diffuse osteopenia and is over-represented in a number of joint registries among patients who have sustained a periprosthetic femoral fracture.36,37,40 Patients with a femoral neck fracture who were treated with THA are also at increased risk. The Finnish Registry showed that patients who had sustained a previous hip fracture had twice the risk of a periprosthetic fracture compared with patients who had rheumatoid arthritis40; similar observations were made from the Swedish Registry.36 This increased risk could well be due to poor bone stock, as is present in most patients who have sustained a femoral neck fracture.
Osteolysis also has a role to play in late periprosthetic fractures.14,34,44 Understanding the pathophysiology is important because it has implications for surgical management of the fracture. Not only must the problem of deficient bone be addressed, as well as the possibility of a loose implant; the issue of the source of wear particles often necessitates revision of bearing surfaces.29,42
Aseptic loosening as a risk factor for periprosthetic fracture34,45 is often the end result of osteolysis secondary to wear particles, causing bone resorption. This is further exacerbated in loose cemented stems by motion at the cement-bone interface.10 Evidence of loosening before a fracture occurs has been reported in a large number of cases. Bethea and coworkers observed in their series that 75% of patients showed evidence of loosening before fracture.10 Others have reported that almost half of the patients they reviewed demonstrated evidence of loosening.11,46 This is supported by one of the largest reviews to date, which demonstrated, in a series of more than 1000 fractures, that 70% were loose before the injury occurred.39
Revision surgery is associated with both intraoperative and postoperative periprosthetic fractures.25,36,47 Poor bone stock is probably the most significant factor in this setting, but the number of revisions also has a role in fracture development.36 Furthermore, the time between a revision operation and occurrence of fracture appears to diminish with each additional revision surgery.39
Various intraoperative factors related to surgical technique can precipitate a fracture. Cortical perforations, such as screw holes, previous hardware, osteotomies, or perforations caused by cement removal or reaming, may result in a stress riser.44,48 Other risk factors for intraoperative cortical perforation include osteoporosis, poor bone stock, and a narrow medullary canal.15 Cortical defects can have a significant impact on cortical strength. Animal studies have shown that torsional strength can be adversely affected by 44%,49 although bypassing the defect by 2 diaphyseal diameters with a long stem can improve bone strength to 84% of the intact contralateral femur. Clinical studies seem to suggest a causal relationship between localized areas of weakened bone and periprosthetic fracture.2,5,10,50 Meek and associates26 support this observation. They found that patients with a low ratio of cortical width to femoral diameter were at greatest risk for a diaphyseal fracture. Box 102-1 summarizes the risk factors for periprosthetic hip fracture.
Prevention
Prevention of periprosthetic fractures starts from the time of surgery. Particularly in revision surgery, it has been shown that exposure is key to avoidance of complications including periprosthetic fracture. A number of extensile techniques have been developed to help reduce the risk of intraoperative fracture.51 In the revision setting, a fracture can occur during hip dislocation, cement and implant removal, reaming of the acetabulum and femur, trial reduction, or during final insertion of the implant.2,7,25,52 During hip dislocation, the risk of fracture is higher in patients who have had previous surgery such as a plate and screws for a femoral fracture, because of stress risers that occur at the old screw holes and stiffness from the prior surgical procedure. One option to help reduce the risk of fracture is to dislocate the hip first if necessary then relocate, before removing the plate.
Patient anatomy may also predispose patients to fracture. From a review of preoperative radiographs, it can be seen that if the trochanter is overhanging the canal in the primary setting or the stem in a revision setting, it would be prudent to provide sufficient clearance of the overhanging bone to allow instrumentation of the femur or removal of a stem without fracturing the greater trochanter (Fig. 102-1). This can be accomplished by creating a channel, or by completing a standard or extended trochanteric osteotomy. Risk of fracture during cement removal can be reduced by adequate visualization of the proximal femur; this can be achieved by performing a standard or extended trochanteric osteotomy.53

Figure 102-1 Anteroposterior (AP) pelvis radiograph demonstrating a varus deformity of the femur and the greater trochanter overlying the medullary canal, requiring modified exposure to correct the deformity and avoid damage to the trochanter.
As highlighted by Mitchell and associates,54 an extended trochanteric osteotomy allows wide exposure, easier cement removal, correct location of the distal medullary canal so as to reduce the risk of cortical perforation, accurate alignment of the revision stem, and correction of the deformity, if required. A cortical window or a controlled perforation of the anterior cortex is another option to assist with implant or cement removal. Sydney and colleagues55 described a very low rate of subsequent periprosthetic fracture with the latter technique, as long as the perforation is made anteriorly and then is bypassed with a long cementless stem by two diaphyseal diameters. This should be by a minimum distance of two bone diameters at the level of the cortical defect. Another simple measure to help prevent intraoperative fracture in revision surgery is to prepare the acetabulum before the femur. This practice not only reduces blood loss, it also minimizes the risk of weakening the femur and the possibility of fracture during retraction.
Surgeons should be wary of unrecognized cortical perforation at the time of a revision that can be unintentionally made larger during canal preparation; this can predispose the femur to fracture or can create a “false passage” and misplacement of the stem tip outside the canal. The use of guide wires as well as intraoperative fluoroscopy or plain radiographs can help avert such an error.
Excessive force should be avoided when reamers, rasps, or the definitive implant is introduced into the femur. The femoral canal should be meticulously cleared of debris using crochet hooks before femoral reaming; crochet hooks can also act as an extension of the surgeon’s hand to ensure that a cortical perforation or a residual pedestal does not exist. Reaming initially by hand or with flexible reamers assists with sizing of the canal and further ensures central positioning within the canal. Further, if an extended trochanteric osteotomy is used, placement of a prophylactic cerclage wire distal to the osteotomy before manipulation of the canal can help to protect the femur from hoop stresses associated with reaming, broaching, and final implant insertion. If considerable resistance to insertion of the broach, trial implant, or final implant occurs, it is advisable to stop and obtain an intraoperative radiograph to confirm appropriate placement.
The danger of under-reaming on the acetabular side has already been highlighted. Similarly, under-reaming on the femoral side is hazardous, particularly if the bone is osteoporotic. If a straight stem is being used, under-reaming by 0.5 mm and no more54 is considered safe. It is important to recognize that the labeled size of the final implant may vary from the size reported by the manufacturer, and the use of a hole gauge can be invaluable in ensuring that the amount of intended under-reaming is accurate (Fig. 102-2). Ideally these gauges should be metallic in construction, rather than the commonly supplied plastic varieties, because the plastic can warp during autoclaving, leading to the risk of mismeasurement.
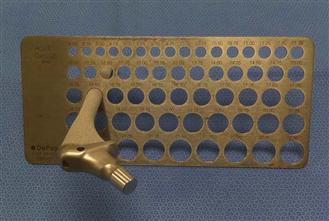
Figure 102-2 Photograph illustrating the use of a hole gauge with a fully porous-coated stem. In this case, the labeled implant size was 15 mm, but measurement of the stem showed it to be 15.5 mm. The femur was subsequently reamed to 15 mm to obtain an appropriate press-fit without increasing the risk of a periprosthetic femur fracture.
If cortical defects or osteolytic lesions are present near the tip of the stem, they can be grafted with cortical strut grafts as a prophylactic measure or bypassed with a long stem.* Large osteolytic lesions of the greater trochanter should be packed with bone graft after granulomatous tissue has been excised. If there is any doubt as to the integrity of the greater trochanter, it should be prophylactically reinforced with a cerclage wire or a claw and cable plate system to prevent crack initiation and propagation.58,59 An intraoperative radiograph should be considered if a fracture is suspected. Finally, postoperative films, including a full-length view of the stem, should be routine, and postoperative management can be modified if necessary.
Anticipation and prevention of intraoperative fractures should form an essential part of preoperative planning. Being aware of possible stress risers in the femur and taking great care with cement removal are essential in avoiding this complication. Postoperatively, the risk of periprosthetic fracture can be reduced by careful supervision and assessment. Clear instructions should be given as to the patient’s weight-bearing status and level of activity, and it should be ensured that the patient has suitable accommodations at the time of discharge. Any condition that predisposes to a fall should be identified and treated.
Careful clinical and radiographic follow-up of patients should help in preventing late periprosthetic fractures.33,36 Radiographs should be assessed for evidence of early osteolysis and aseptic loosening, and if a failing hip arthroplasty is identified, it should be revised.44 It is difficult to know when to intervene, particularly if the patient is asymptomatic; however, progressive bone loss should be considered as an indication to intervene so as to prevent fracture.33
Diagnosis
The diagnosis of periprosthetic fracture is based on history, as well as on physical and radiographic examinations. Fractures generally can be recognized intraoperatively by direct observation or by radiographs. This is likely to occur during one of the three stages of reconstruction: previous implant removal, bone preparation, or placement of the revision implant. Signs that alert the surgeon to this possibility include a change in pitch while using instruments, a sudden reduction in the force required when a broach or rasp is used, or advancement of the implant beyond the planned level of placement, either in the socket or in the canal.
Postoperative periprosthetic fractures are typically associated with an acute onset of pain and deformity that may or may not follow a history of a fall. However, clinical suspicion of a periprosthetic fracture is necessary in patients with nondescript complaints of pain around their implant, especially if osteolysis is evident. In a review by the Mayo Clinic,33 as many as 50% of patients with fractures did not have any precipitating traumatic event. Researchers attributed these fractures to implant loosening or osteolysis and highlighted the importance of regular radiographic follow-up of all patients with primary and revision THAs.
Radiographic evaluation of all suspected fractures should be comprehensive, including full-length views of the femur in two planes, an anteroposterior pelvic radiograph, and Judet views to more completely evaluate the floor, roof, and columns of the acetabulum. Comparison with previous radiographs is also helpful. More specialized imaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), has not yet been accepted as standard practice because of difficulty with metal artifact, inconvenience, expense, and unproven cost-benefit. However, these tests may be helpful in selected circumstances of unusual complexity, especially around the acetabulum, and can be combined with three-dimensional reconstruction to assist with preoperative planning. Software packages have been developed for use with CT scanners to help reduce metal artifact, and improvements have been reported with MRI as well.60,61 Bone scans have been used in the past, but increased uptake is a nonspecific finding; thus their use has been largely superseded by the use of metal artifact–reducing CT scans.
Classification
Undoubtedly the best outcome in the management of periprosthetic fractures is achieved when the surgeon has a thorough understanding of the problem and is appropriately prepared with the necessary implants and instruments. A classification system that combines the anatomy and biomechanics of the fracture type with proven principles of treatment that will lead to the best outcome is critical in achieving this level of understanding and preparedness.
Acetabular Fractures
The classification of periprosthetic acetabular fractures is based on the system developed by Letournel.62 By defining the anterior and posterior columns, Letournel formed the basis for describing fracture patterns as seen on three plain radiographs: an anteroposterior pelvic view and two 45-degree Judet oblique views. Subsequently, the classification was modified,63,64 with elementary fractures including posterior wall, posterior column, anterior wall, anterior column, and transverse injuries, and associated fractures including both columns, transverse plus posterior wall, anterior wall/column plus posterior hemitransverse, posterior column plus posterior wall, and T-shaped injuries.
Callaghan and coworkers65 used a cadaveric model to describe the pattern of acetabular fractures following impaction of acetabular components in under-reamed acetabula. They included anterior wall, transverse, inferior lip, and posterior wall fractures. Peterson16 modified Letournel’s62–64 system to include fractures of the medial wall. Fractures were also classified according to the stability of the acetabular component: type 1 if stable, and type 2 if not.
Pelvic discontinuity is important to recognize because standard acetabular revision techniques may not be adequate for reconstruction. Berry and associates4 described the diagnostic features of pelvic discontinuity in their review of 27 patients. On the anteroposterior radiograph, three cardinal features included an obvious fracture line, malalignment of the upper and lower halves of the hemipelvis (usually due to medial displacement of the inferior half), and rotation of the inferior half of the hemipelvis in comparison with the opposite side (observed by comparing the size and shape of the obturator foramina; Fig. 102-3). Investigators emphasized that Judet views can be helpful when implants obscure the normal acetabular anatomy, especially the columns. In some cases, it is still difficult to diagnose a pelvic discontinuity on plain radiographs, and it in this circumstance that a metal artifact–reducing CT scan may be helpful. Furthermore, in every case, it is important to confirm the continuity of the hemipelvis during the revision procedure, regardless of preoperative radiographic findings.
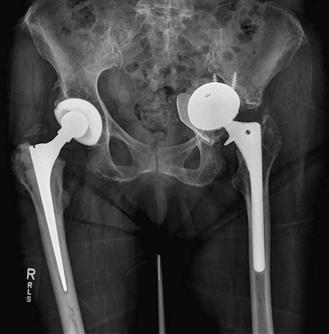
Figure 102-3 Anteroposterior (AP) pelvis radiograph illustrating pelvic dissociation with an absent posterior column, a medially displaced inferior half of the hemipelvis, and asymmetric obturator foramina.
Femoral Fractures
Successful management of these fractures depends on a number of factors, including fracture location and configuration, stability of the implant, quality of host bone, patient physiology and age, and surgeons’ experience in managing these fractures.28 A number of classification schemes have been described,5,6,25,66,67 but the most widely used is the Vancouver classification.68 This system is based on the three most important factors that influence outcome: the site of the fracture, the stability of the stem, and the quality of available bone for reconstruction. This system has been validated in two different studies.69,70
The three major anatomic types defined by this classification system depend on the location of the fracture: Type A refers to the trochanteric area (one or both trochanters and proximal metaphysis, not extending to the diaphysis); type B refers to a fracture around or just distal to the femoral stem (diaphysis); and type C refers to fractures that are sufficiently distal to the tip of the stem that their treatment can be considered independent of the presence of a total hip arthroplasty (the distal diaphysis and/or metaphysis).
Type A is further subdivided into greater trochanter (AG; Fig. 102-4) and lesser trochanter (AL). Type B is also subdivided into those where the implant is stable (B1; Fig. 102-5) and those where the implant is loose (B2; Fig. 102-6). If a fracture occurs through the cement mantle distal to an otherwise well-fixed cemented stem, this too would be classified as a B1. Much more commonly, the fracture passes through the cement mantle around the stem, leading to a B2 fracture, whether or not the stem was well fixed before the fracture. In both of these categories, the bone stock is good. If the femoral bone stock is poor and the implant is loose, the fracture is categorized as a B3 (Fig. 102-7). An example of a type C fracture is illustrated in Figure 102-8.
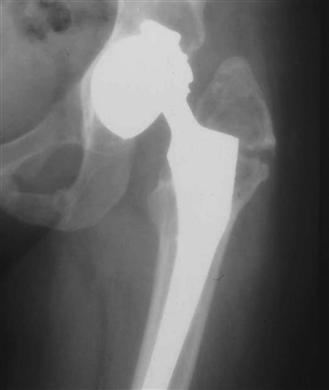
Figure 102-4 Anteroposterior (AP) femoral radiograph demonstrating a Vancouver AG periprosthetic fracture of the femur secondary to wear debris osteolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








