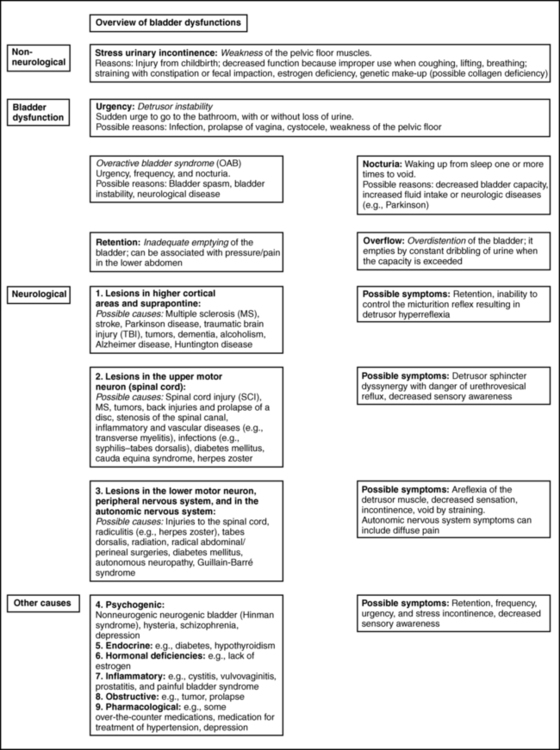
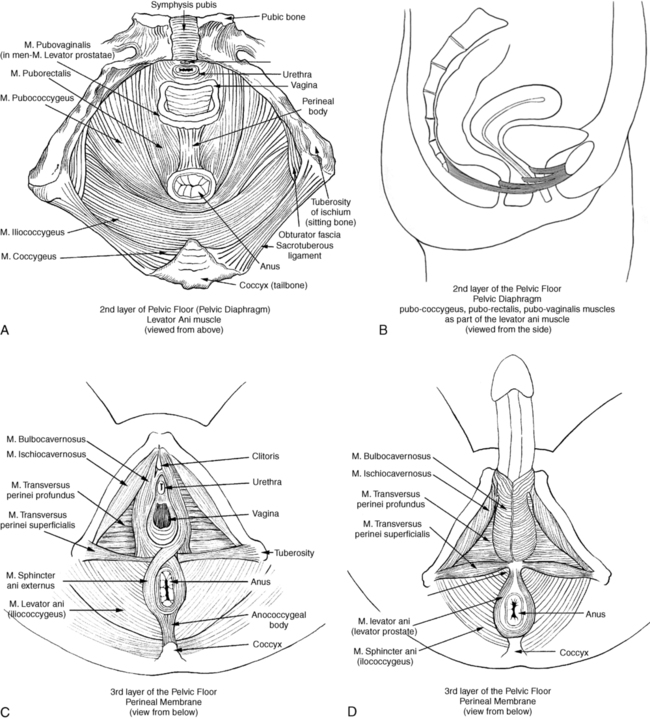
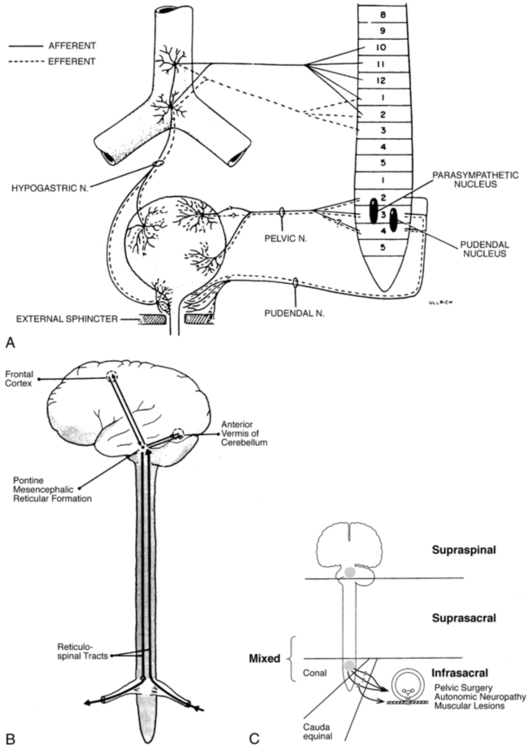
BEATE CARRIÈRE, PT, CAPP, CIFK After reading this chapter the student or therapist will be able to: 1. Discuss factors that lead to incontinence. 2. Understand the neurophysiology involved in pelvic floor dysfunctions. 3. Describe the different layers of the pelvic floor and their functional connections. 4. Understand the importance of coordinating diaphragmatic breathing with pelvic floor activity. 5. Correct faulty breathing patterns to facilitate normal pelvic floor activity. 6. Apply motor learning and motor control principles when teaching exercises. 7. Select the most appropriate intervention to improve pelvic floor function. A different focus on how to view the pelvic floor and the problem of incontinence has evolved from new knowledge about neurophysiology, neuroplasticity, motor learning, motor control, and functional brain imaging.1,2 Kegel3–5 is considered the great American pioneer who, in the late 1940s, recognized the importance of exercises to help women with urinary incontinence (UI). Dr. Kegel, a Los Angeles physician, found that many women did not have any awareness of the function of the pelvic floor and that they were not always successful with the exercise he prescribed, which is drawing in the perineum. He therefore developed a pneumatic apparatus, the perineometer, which measured each muscle contraction in a manner visible to the patient. He instructed his patients to perform the exercise for 20 minutes three times daily, or a total of 300 contractions, and suggested weekly visits for instruction.3,4 Kegel also recognized that evidence of bladder weakness was present in some women before childbearing and emphasized the importance of training the pubococcygeus muscle to achieve continence.4,5 Considered visionary at that time, his approach to exercises demonstrates visual feedback combined with declarative learning. However, the treatment has not changed much since.6–9 Kegel exercises are stereotypical and highly repetitive (300 repetitions until improvement, then 80 per day for life) with little functional value. More recent studies have investigated the effectiveness of Kegel’s exercises. The prevalence of UI in men and women is high and costly. Mardon and colleagues,10 who conducted a study of managed care beneficiaries, found the overall incidence of UI to be consistent with previous estimates. Fantl and colleagues11 reported in 1996 that 10% to 35%, or 13 million adult Americans, have UI. In 2000 Hu and co-workers12 estimated that 17 million community-dwelling persons had daily UI and 34 million had overactive bladder (OAB) syndrome, with 2.9 million of those reported to have had incontinence episodes. In 1996 more than half of the 1.5 million nursing home residents in the United States were estimated to be incontinent. This condition is reported to be the most common reason for placing a family member in a home.11–13 The number of adults in institutional care had risen to 1.89 million in 2000, and 945,000 of those were estimated to have UI.12 Women older than 60 years have twice the prevalence of incontinence as men of that age.11 The majority of patients with incontinence are parous women and older persons.13 Goode and colleagues14 report that UI is a common geriatric syndrome affecting at least one in three older women. “UI is not a normal result of aging, it is a medical problem that is often curable and should be treated.”15 Britton and co-workers16 conducted a study of urinary symptoms in 578 men older than 60 years. Thirty percent of the men reported increased daytime frequency, and 27% reported urgency and a variety of other urinary symptoms, defined as OAB if nocturia (getting up at least once in the night to urinate) is included. Incontinence can also be found in the younger population. Nygaard and colleagues17 investigated UI in nulliparous elite athletes and found that individuals who participate in gymnastics and sports that include jumping, high-impact landings, and running appear to score higher in the prevalence of UI than those who swim or play golf. Because exercises and activities during field training of soldiers can be strenuous, one third of 450 female soldiers experienced UI according to Sherman and Davis.18 Baumann and Tauber19 reported that 17% of boys aged 5 to 14 years are incontinent. Adedokun and Wilson20 and Diokno and colleagues21 provided thorough epidemiological overviews, collecting data from various sources all over the world. Risk factors for incontinence include the following: Obesity: This is a risk factor and can contribute to incontinence and make the treatment more difficult.22,23 Cigarette smoking: Smoking augments incontinence for three reasons: (1) it has been shown to interfere with collagen synthesis, (2) neuromuscular and anatomical changes likely occur from smoking that result in decreased functionality of the bladder, and (3) it causes coughing, which increases the strain on the weak pelvic floor muscles.24 Moreover, cigarette smoking has been linked to erectile dysfunction and has an effect on sperm quality in men. In women, it is related to increased incidence of miscarriage and cervical cancer and reduced chance of conception.25 It has been reported that smoking cessation, weight reduction, and regulation of bowel movements may reduce the risk of UI.26 Diabetes: Hunter and Moore27 state that an estimated 13% of seniors have diabetes. Of these individuals, 32% to 45% have associated bladder dysfunction. The presence of UI in diabetics can be attributed to decreased bladder sensation, increased bladder capacity, and impaired detrusor contractility. Lee and colleagues28 studied voiding patterns in women with type 2 diabetes and stated that peripheral neuropathy was an important factor associated with diabetic voiding dysfunction. They found that patients with diabetes had predominantly nocturia and a weak urinary stream, abnormal nocturnal urine production, and decreased voiding volumes and functional bladder capacity. In the same study, researchers noted impaired detrusor contractility, with 13.9% of 194 female patients having high postvoiding residual (PVR) urine of over 100 mL. The researchers suspected that the dysfunction in detrusor contractility was secondary to diabetes. Moreover, 14.4% of the women in the study had less than 70% efficiency of emptying the bladder. Neurologic diseases associated with UI include the following: Brain injuries: Leary29 investigated incontinence after brain injury and reported that 50% of the patients had impaired bladder and bladder subscores on admission. Even though 90% of those patients were set goals for self-care and mobility, only 3.5% of the patients were set multidisciplinary goals addressing bladder and bowel function. The Agency for Health Care Policy and Research30 reported that UI is most prevalent in persons with spinal cord injuries (SCIs) and people with multiple sclerosis (MS). Eighty percent of patients with SCI will have had at least one urinary tract infection (UTI) by their sixteenth year postinjury. MS: Seventy percent to 90% of individuals diagnosed with MS develop bladder dysfunction, which places them at high risk for UTIs (Agency for Health Care Policy and Research). McClurg and colleagues31 state that in patients with MS, bladder storage problems often coexist with inadequate emptying of the bladder because of detrusor sphincter dyssynergy. In their research with 30 patients with MS, they showed that a combined treatment of pelvic floor training and advice combined with electromyography biofeedback and neuromuscular electrical stimulation may reduce urinary symptoms in these patients. Schulte-Baukloh and colleagues32 injected 11 women and five men with MS and drug-resistant OAB symptoms with botulinum toxin type A and found significant improvement for 4 weeks to 3 months (but not at 6 months), with reduction in frequency, nocturia, and pad use; however, buildup of residual urine remained a problem. Spina bifida: Patients affected by spina bifida also can have various neurogenic urinary tract dysfunctions. Depending on the severity of the dysfunction, they may become socially dry with conservative therapy.33 Alzheimer disease: In patients with Alzheimer disease cognitive and central regulating mechanisms contribute to incontinence.34 According to Holstege,1 research has shown that “lesions in the pathway from prefrontal cortex and limbic system, to the PAG (periaqueductal gray) probably cause urge incontinence in the elderly.” Cerebrovascular accident (CVA): Brittain and colleagues35 state that UI after a stroke is associated with poor outcome and depression in stroke survival and care. The overall prevalence of UI is high (32% to 79%) in older patients with stroke admitted to the hospital. At discharge the incidence of UI is 25% to 28%, and 12% to 19% continue to experience UI for months after the stroke. The inability to communicate, transfer, or walk and the use of drugs complicate matters. UI in stroke patients is similar to UI in patients without stroke and probably results from muscle weakness on the affected side. The patients may demonstrate stress UI (SUI), but urgency UI (UUI) and mixed incontinence are also common. Studies published from 1985 to 1997 suggest that 31% to 40% of hospitalized stroke patients have fecal incontinence on admission and 18% at discharge, and 7% to 9% still experience fecal incontinence 6 months after the stroke.36 Unfortunately, treatment for these problems is rarely prescribed, even though it is very effective.37 Parkinson disease: In individuals with Parkinson disease, the prevalence of lower urinary tract symptoms (LUTSs) is 27% to 39%, most frequently nocturia (86%); 71% of patients have reported frequency, and 68% have reported urgency. According to a study by Winge and colleagues,38 the patients were bothered most by the urgency symptoms. The authors inferred that the reason could be a progression of gait difficulties, decreased ability to separate and integrate sensory input, or both at a later stage of the disease. Zein and colleagues39 agreed with increased incidence of voiding dysfunction with the progression of the disease. They also describe incomplete emptying (40%) and hesitation (37.3%) as problems in their prospective study of 110 patients with Parkinson disease. Fowler and Griffiths2 suggest that the pathways from the PAG to the thalamus and insula do not conduct signals properly in Parkinson disease. Deep brain stimulation can restore the conduction. Parkinsonian syndrome: Multiple system atrophy (MSA) is described in detail by Yamamoto and colleagues39a and Wenning and colleagues,39b who state that the clients have autonomic, cerebellar, and extrapyramidal symptoms. The syndrome presents as a more severe form of Parkinson disease and includes MSA, idiopathic Parkinson’s, vascular parkinsonism, and supranuclear palsy. Clients with MSA can have severe blood pressure variations and sudden orthostatic hypotension secondary to autonomic system problems in addition to more severe bladder dysfunctions such as higher incidence for urinary urgency, retardation in initiating urination and incomplete emptying of the bladder that may require catheterization. Prolongation of urination and constipation are also more common in MSA clients than those with Parkinson disease. The physical treatment has to address more symptoms and it is more complex and more difficult to improve the client’s quality of life. Guillain-Barré syndrome (GBS): The prevalence and mechanism of bladder dysfunction were evaluated by Sakakibara and colleagues.40 In their study of 65 consecutive patients urinary dysfunction was observed in 27.7%. Of those patients, 9.2% experienced retention, 24.6% had voiding difficulties, and 7.7% complained of urgencies. Overactive and underactive detrusor activity were the major urodynamic findings. The underlying mechanism, according to the authors, appeared to involve hypoactive and hyperactive lumbosacral nerves. Wilson and colleagues41 state that the cost for women over 65 years of age with UI is twice that for women younger than 65 years. Affecting 17% to 55% of community dwellers and up to 50% of nursing home residents, UI is one of the most prevalent chronic diseases. Only one-quarter to one-half of affected individuals seek medical attention. Within a 10-year period the direct cost increased considerably, more than could be accounted for by medical inflation. Hu and colleagues12 adjusted and reported the cost of UI and OAB syndrome in 2000: $19.5 billion and $12.6 billion, respectively. Thirty-four million individuals had OAB syndrome, and 17 million had UI. Because individuals with OAB have fewer incontinence episodes, the per-person cost is higher in patients with UI—35% of patients with UI have SUI, which can involve expensive surgeries. However, the days spent in the hospital have declined since 1995.12 The cost of UI escalated from $8.2 billion in 1984 to $16.4 billion in 1993 and to $26.3 billion, or $3565 per individual with UI, in 1995. Wagner and Hu42 attributed this increase to the following three major changes in the previous 10 years: UI is defined as involuntary loss of urine.43 Incontinence can have one or more causes, and in more than 90% of cases it can be improved or cured.13 Treatment should be instituted only after a careful, thorough history and physical examination.13 SUI is defined as involuntary loss of urine on effort or physical exertion, for example, sporting activities or sneezing or coughing.43 UUI is considered to be involuntary loss of urine associated with urgency.43 Mixed UI is the complaint of involuntary loss of urine associated with urgency and also with effort or physical exertion, sneezing, or coughing.43 Symptoms associated with bladder storage disorders include the following: Increased daytime urinary frequency: Micturition (emptying of the bladder) occurs more frequently during waking hours than previously deemed normal by the patient.43 Nocturia: Patient wakes up from sleep one or more times because of the need to micturate. Each void is preceded and followed by sleep.43 Urgency: A sudden, compelling desire to pass urine that is difficult to defer.43 OAB syndrome: Cluster of symptoms of urinary urgency, usually accompanied by frequency and nocturia in the absence of UTI.43 Symptoms associated with voiding disorders include the following: Hesitancy: Delay in initiating micturition43 Straining to void: A need to make an intensive effort to either maintain or improve the urinary stream43 Feeling of incomplete bladder emptying: Report that the bladder does not feel empty after micturition43 Urinary retention: Inability to pass urine despite persistent effort43 In patients with nonneurological conditions, the most common forms of incontinence are SUI, OAB syndrome, UUI, and mixed incontinence. Weak pelvic floor muscles usually cause SUI. SUI occurs when the abdominal pressure is greater than the urethral pressure, resulting in a loss of urine with coughing, laughing, sneezing, lifting, and so forth. OAB syndrome, or detrusor overactivity, is associated with involuntary bladder muscle contraction during the filling phase, causing frequent urination; nocturia; and strong, sudden, and sometimes unpredictable urges to urinate but not always resulting in incontinence.44 The causes of SUI and UUI in individuals with nonneurological conditions are as follows (Figure 29-1): 1. Functional causes include the inability to undress in a timely fashion and not being able to reach the bathroom in a timely fashion because of obstacles (e.g., no light, cannot enter the bathroom with the walker without maneuvering). 2. Weakening of the pelvic floor structures can result from childbirth (overstretching of muscles, injuries to the pudendal nerve, or ligaments), hysterectomy, prolapse (rectocele, cystocele, vaginal prolapse), straining with constipation, poor biomechanics when lifting, falls on the buttocks with shift of the pelvis affecting muscle length or stretching the nerves, and poor coordination of the pulmonary diaphragm with the pelvic floor, back, and abdominal muscles.45,46 Scars in the perineal and pelvic area, aging (loss of muscle mass), obesity, smoking, poor posture, and pain in the pelvic area also can contribute to muscle weakness. 3. OAB syndrome and UUI can be caused by UTIs and other conditions that irritate the bladder: neoplasia, post–bladder or post–bowel surgery status, bladder outlet obstruction, anxiety, nervousness, and poor toileting habits. The condition can also be idiopathic. Some clients also have urgency to eliminate the bowels. 4. Over-the-counter medications with anticholinergic agents can cause retention (an inability to empty the bladder completely), overflow (leakage of urine when the bladder is overextended), and frequency; antipsychotic medications can cause sedation, rigidity, and immobility; and diuretics can worsen impaired continence. Medication for treatment of hypertension can also contribute to incontinence.11,13,27,47,48 5. Retention in nonneurological clients can occur in men with prostate problems; the enlarged prostate makes the passage of urine difficult.16,49 Other causes in men and women are the hyperactive pelvic floor syndrome (HPFS),50 an inability to relax the pelvic floor muscles. This can be caused by pelvic pain syndromes, by painful bladder syndrome (formerly interstitial cystitis), or by habits: trying to void in a hurry, squeezing rather than relaxing the muscles, and being unable to void because of stressful situations. 6. An overdistended bladder can cause overflow incontinence. It can manifest as constant or intermittent dribbling, sometimes combined with urgency or symptoms of stress incontinence. Patients often have high residual urine levels and feel that their bladder does not empty properly; sometimes secondary to sensory problems, the patients are unaware of the filling of the bladder. 7. Inadequate fluid intake (either too much or too little) and fluids that may be stimulants to the bladder can cause urgency incontinence or frequent trips to the bathroom. Smoking, obesity, and postmenopausal estrogen deficiency contribute to the problem.11,22,24,47,48 Neurogenic causes of bladder dysfunctions are as follows: 1. Lesions in the higher cortical areas and suprapontine lesions can be found in patients with MS, stroke, Alzheimer disease, Parkinson disease, traumatic brain injury, tumors, and dementia. Lateral prefrontal cortex activation probably regulates the desire to void and to remain continent.2 Lesions in the pathways from the prefrontal cortex and limbic system to the PAG probably cause urgency incontinence in the elderly.1 The emotional motor system (EMS) uses the cell group of the pontine micturition center (PMC), close to the locus coeruleus, which sends long descending fibers to the parasympathetic motoneurons to the sacral cord. Through inhibitory interneurons, fibers reach the nucleus of Onuf. Stimulation of the PMC therefore results in complete micturition.1 Neurological diseases can cause many other symptoms such as retention and an inability to control the micturition reflex. Detrusor hyperreflexia (overactive detrusor during the filling phase) with coordinated urethral relaxation interferes with normal tonic inhibition of the parasympathetic pathways and the balance between facilitatory and inhibitory mechanisms in the PMC.44,47,48,51 2. Lesions in the upper motor neurons (UMNs) affect the spinal cord. Common problems resulting in urinary dysfunction are SCI, MS, cauda equina syndrome, tumors, inflammatory diseases such as transverse myelitis, infectious diseases such as syphilis (tabes dorsalis), injuries to the spinal column, prolapse of a disc, or stenosis of the spinal canal. A typical bladder problem is detrusor hyperreflexia without coordinated urethral relaxation (detrusor-sphincter dyssynergy).47,48,51,52 3. Lesions of the peripheral or lower motor neurons (LMNs) such as injuries from childbirth, traumatic injuries, diabetes, radiculitis (e.g., from herpes zoster), or tabes dorsalis can cause retention and detrusor areflexia. The inability to feel when the bladder is full can lead to an overflow bladder with symptoms of dribbling and incomplete or strained voiding. Patients may need to learn clean intermittent catheterization.11,13,48,51,53 4. Lesions stemming from injuries to the autonomic nervous system can be caused by surgery in the pelvic area, such as hysterectomy, rectum resection, and radical prostatectomy; injury; or inflammations such as chronic cystopathy in diabetic patients. Autonomic lesions can contribute to diffuse pain,54 swelling, and altered sensory awareness. Patients with urinary problems can describe the feeling of having cold feet.55,56 Patients with diabetic cystopathy (DC)—in addition to their symptoms of weak stream, hesitancy in starting urination, dribbling, and overflow from high residual urine levels (caused by decreased bladder sensation and increased bladder capacity, and impaired contractility)—may also have other symptoms of autonomic dysfunction. These can include orthostatic hypotension, nocturnal fall of blood pressure, and changes in heart rate.7,27 Altered central nervous system (CNS) monoamines (serotonin and noradrenaline) can cause both depression and OAB. Correction of some neurologic disorders can eliminate both depression and urgency incontinence.57 5. Psychogenic causes of urinary dysfunction include schizophrenia and depression. Patients can experience incontinence, hesitation, retention, and pain. 6. A nonneurogenic neurogenic bladder is called Hinman syndrome.47 7. Endocrine causes are hypothyroidism and diabetes, which may lead to a flaccid or areflexic bladder and require clean intermittent self-catheterization.47,53 Diabetes can also increase the frequency of urination. The pelvic floor consists of all the muscles that close the pelvic cavity. It is part of the abdominal compartment that can be defined by the pulmonary diaphragm cranially, the pelvic diaphragm and perineal membrane caudally, the muscles of the abdominal wall ventrally, and the muscles of the back dorsally. This compartment houses the internal organs and the viscera.13,45–47,58–61 All muscles of the abdominal compartment interact and support one another, providing postural stability and the ability to breathe, talk, and eliminate. The pelvic floor is essentially composed of three layers: the endopelvic fascia, the pelvic diaphragm, and the perineal membrane. The endopelvic fascia suspends and supports the organs within the pelvis. It is a mesh of connective tissue composed of collagen, elastin, blood and lymph vessels, and nerves. A thick, fibrous part of the endopelvic fascia, the pubocervical fascia, attaches to the cervix in a slinglike fashion and assists in supporting the urethra and the bladder. Laterally it connects to the fascia white line, the tendinous arch of the levator ani muscle. Injuries to this important fascial support can contribute to weakness of the pelvic floor, prolapse, and leakage with increased abdominal pressure.13,48,61 One study62 has shown that fascia may be able to contract in a smooth muscle-like manner and therefore may have the ability to influence musculoskeletal dynamics. This underlines the importance to not neglect the fascial structures surrounding the abdomen when treating pelvic floor dysfunctions. They are not purely passive structures. The pelvic diaphragm consists mostly of the paired levator ani muscle (Figure 29-2). The levator ani is shaped like a hammock and has several parts. In the sagittal plane the muscle originates at the pubic bone and attaches to the coccyx, hence the name pubococcygeus muscle. The medial part of the pubococcygeus joins behind the rectum and therefore is named the puborectalis muscle. This part of the levator muscle provides continence of bowel by increasing the anorectal angle. Inability to relax the puborectalis therefore contributes to constipation in patients with HPFS. Other fibers of the levator ani form a sling around the vagina or prostate (pubovaginalis and levator prostate muscles). Each side of the muscle meets in the midline with the other half and attaches to the perineal and anococcygeal bodies. The compressor urethrae muscle is part of the external urethral complex. It originates from the rami of the ischium and pubis and runs fanwise forward and medially to arch over the anterior part of the urethral surface. The sphincter urethrovaginalis is part of the puborectalis.63 The posterior part of the levator ani has two paired sections. The coccygeus (or ischiococcygeus) muscle covers the sacrospinous ligament. The muscle arises at the spine of the ischium and extends to the lowest part of the sacrum and the coccyx. The iliococcygeus lies between the coccygeus and the puborectalis and passes in a diagonal direction between the coccyx to the spine of the ischium and the tendinous arch of the levator ani. This fibrous band of the arcus tendineus is suspended between the pubic bone and ischial spine.13,47,48,61,63–65 The levator ani is a skeletal muscle with a high resting tone; it consists of approximately 70% slow-twitch fibers and 30% fast-twitch fibers.48,61,66–68 Wall and colleagues48 and Bump and colleagues66 consider the high resting tone critical for pelvic support and for keeping the hiatus of the levator closed. Because the levator ani muscle is under voluntary control, it can be actively contracted and provide closure during an increase in abdominal pressure, such as when coughing or sneezing.48,67–69 According to Retzky and Rogers,13 the innervation of the levator ani muscles is under dual control: on the pelvic surface by motor efferents of the sacral nerve from S2 to S4, and on the perineal surface by the pudendal nerve. The perineal membrane (formerly urogenital diaphragm) is the outer layer of the pelvic floor. It is a thick, fibrous, and muscular layer of triangular shape immediately below the levator ani. In women the perineal membrane attaches the edges of the vagina to the ischiopubic ramus; in men it forms an uninterrupted sheet of tissue. The fibers of the deep and superficial transverse perineal muscles run primarily in a frontal plane and contain many fibrous tissues,70 the ischiocavernosus muscle in a diagonal direction, and the bulbospongiosus muscle in a sagittal plane. The external sphincter muscle of the anus is part of the perineal membrane and is connected to the transverse perineal and bulbospongiosus muscle by the perineal body, which contains fibrous tissue. The dorsal attachment of the perineal membrane is achieved through the anococcygeal raphe, which connects the external anal sphincter (EAS) to the coccyx.48,64,67,70 The muscles of the perineal membrane include both smooth and striated muscles. The muscles become tight when the levator ani tone remains relaxed.48 The anterior portion of the perineal membrane is closely connected to the urethral musculature. According to Wall and colleagues,48 the perineal membrane does not substantially contribute to pelvic support; it is mostly the levator ani, which has much greater strength and bulk and can exert upward traction when contracting to maintain outlet support. The ischiocavernosus, bulbospongiosus, and superficial transverse perineal muscles function mainly in sexual responsiveness, serving to enhance and maintain penile erection in males and maintaining erection of the clitoris in females.48,61 Trigger points in these muscles can cause a degree of impotence and pain with intercourse. According to Claes and colleagues,71 Van Kampen and colleagues,72 and Dorey,73 strengthening exercises of the muscles of the perineal membrane can significantly improve impotence. In women the perineal membrane is often torn or injured during childbirth, and, if not properly repaired, injuries can cause sexual dysfunction, pelvic pain, and low self-esteem. The complicated autonomic innervation of the pelvic area and its clinical relevance, including the perineal area, has been well described by Wesselmann and colleagues54 as well as Fritsch and Umphred.70 The pudendal nerve diverges from the sacral plexus, intermingles with the autonomic nerves, and then branches into several directions, innervating the EAS and the anterior perineal muscles. In addition to the three pelvic floor layers, an important contributor to continence is the mucosal coaptation, which is the arteriovenous complex between the epithelial lining and the smooth muscle coat of the female urethra. It is sensitive to estrogen, and with deficiency of this hormone the resting pressure of the urethra can decrease and cause leakage.13 Wall and colleagues48 compared it with an “inflatable cushion” helping to fill the urethral wall and sealing the 3- to 4-cm–long urethra in women. Because of possible serious side effects of estrogen treatment, it is now prescribed and applied to the vaginal area in low dosage and with caution. It can help some menopausal women increase the resting tone of the urethra and improve closure. The male urethra is not estrogen dependent. Its mucosal coaptation is highly vascular and probably influenced by testosterone. In addition, the prostate gland and the length of the male urethra may contribute to a sealing effect. The internal sphincter muscle at the junction of the bladder and urethra (urethrovesical junction) is an involuntary smooth muscle under autonomic control in both males and females. Its shape is circular and formed by the trigone, a smooth muscle in the bladder, and two U-shaped loops of muscles that derive from the bladder muscle. In females the urethra rests on a hammock of connective tissue (pubocervical fascia) and is held in a position that prevents descent into the vagina. The external sphincter muscle is able to close the middle portion of the female urethra.13 Incontinence usually happens when several factors come together. For example, men frequently leak after radical prostatectomy because the smooth internal sphincter urethra may have been damaged by the surgery and the pelvic floor muscles have to learn to substitute and provide closure of the urethra. A patient with dementia may not feel the need to go to the bathroom because of lesions in the limbic system or the cortex.1,2 Parkinson disease may cause bladder outlet obstruction because of a noncontractile detrusor, resulting in large volumes of residual urine or lack of activation of the PMC.2,74 In addition, the inability to quickly undress complicates an already existing problem of incontinence in such a client. Many of the neurophysiological connections involved in functioning of the bladder and the surrounding muscles are not fully understood. A complicated coordination of many systems is involved in a properly functioning bladder and other pelvic organs. Elaboration on all the neural interactions, which take place at all levels, is not possible in this chapter. Wesselmann and colleagues54 and Burnett and Wesselmann75 provide comprehensive information about the neurobiology of the pelvis. The sympathetic innervation to the bladder, rectum, and sexual organs originates from the thoracolumbar segment of the spinal cord (T10 to L1-L2) as well as from the hypogastric plexus (the sympathetic hypogastric nerve), which descends from the aortic plexus (Figure 29-3). The hypogastric nerve feeds into the inferior hypogastric (pelvic) plexus, which is the major neuronal integrative center for multiple pelvic organs.54,75 Both the sympathetic and parasympathetic divisions of the autonomic nervous system innervate the pelvic viscera, which are also innervated by the somatic and sensory nervous systems. The sympathetic innervation inhibits the bladder and increases bladder storage ability (it is sympathetic to be dry) and stimulates the muscles of the trigone and the internal sphincter muscles of the bladder as well as the muscles of the rectum. Attention should be paid to the T10 to L1-L2 segments in patients with tailbone pain and pain in the pelvic floor (e.g., testicular, buttock, scrotal pain) because the pain may be referred from that area. I recall a case in which tailbone pain disappeared instantly after treatment of the thoracolumbar area in a client who had fallen on the back in a flexed position. Doubleday and colleagues76 described a case of a patient who for 5 years had complained of testicular and buttock pain along with posterior leg paresthesias. Treatment of the T10 to L1-L2 area (central disk protrusion at T12-L1) with direct and guided physical therapy resulted in complete symptom resolution. The parasympathetic innervation exits the spinal canal at the level of S2 to S4. The nerves join the splanchnic nerve before entering the inferior hypogastric plexus (see Figure 29-3, A). The parasympathetic fibers stimulate the bladder and other pelvic organs, including the sexual organs.54,75,77 The parasympathetic fibers ending in the bladder are especially sensitive to overstretching, infection, and fibrosis.47 This explains the frequent urgency to urinate under such conditions. The parasympathetic nerve helps bladder contraction by stimulating muscarinic receptors in the fundus of the bladder. Parasympathetic nerve activation also helps colonic mobility. Therefore in an SCI when LMNs are injured (parasympathetic cell bodies in the cauda equina and conus medullaris), the result can be slowed stool propulsion with areflexic bowel. LMN injury causes constipation and increases the risk of incontinence from lax EAS, as described by Benevento and Sipski.52 In contrast an UMN lesion such as those from MS causes hyperreflexia with no voluntary control over the EAS. The patient cannot relax the EAS to defecate even though reflex coordination and stool propulsion are present. Urinary and fecal retention and constipation are common in such UMN injuries.52 Detrusor sphincter dyssynergia (DSD), caused by impaired coordination between bladder contraction and the external sphincter urethra, can be attributable to hyperreflexia or uncontrolled muscle spasm of the detrusor. This condition is common in patients with SCI52 as well as MS but not in clients with cerebral vascular accident (CVA). The somatic innervation of the pelvic floor comes from the sacral plexus of S2 to S4 (see Figure 29-3, A). The pudendal nerve leaves the spinal cord at S2 to S4 and branches to innervate the striated muscles of the levator ani, the external sphincter muscles of both the urethra and rectum, the labia and clitoris, and the muscles of the perineal membrane. The sacral plexus provides both efferent and afferent innervation; some of the fibers of the pudendal nerve intermingle with the autonomic pelvic nerves.47,54,77 The somatic nerves can modulate the autonomic system. The complexity of the innervation of the pelvis and the influence of neurotransmitters in the bladder wall, as well as the control of higher CNS regulation (see Figure 29-3, B and C), can be appreciated in observing the filling and emptying phases of the bladder. Because of its topographical position the pudendal nerve is susceptible to nerve injury from stretching or compression (Alcock canal) from a fall on the buttocks or from slamming on the brake pedal during a motor vehicle accident (may alter pelvic alignment). Patients with pudendal nerve injury often have no problems when supine or standing but pain when sitting. Childbirth, especially during delivery of large babies, also can cause pudendal nerve injury.13 The sensory innervation in the pelvic region is important to evaluate and restore in clients with motor control problems because sensation drives motor responses. The pudendal nerve carries both sensory and motor fibers. Absence of feeling in the perineal area can be caused by stress and memory of pain (e.g., abuse). For example, a client with symptoms of incontinence and pain with sexual intercourse and a history of considerable continuous stresses stated after few treatments, “I thought my pelvic region was dead; I had no clue how little I could feel before sensory awareness training. Feeling the pelvic floor, I can now contract and relax the muscles much better.” Sensory innervation of the bladder comes primarily from proprioceptive nerve endings in the bladder and urethra. The afferent neuronal visceral system probably enters the spinal cord by way of the sacral and lumbar segments. These afferent nerve tracts may regulate pain, the absence of pain, and the feeling of having a full bladder.47,77 The bladder is a smooth involuntary muscle with voluntary control. It stores the urine until it is emptied voluntarily. The normal bladder is a low-pressure system that accepts urine without a concomitant rise of internal pressure.13,48 This is produced by sympathetic stimulation of the β-adrenergic receptors in the bladder wall. The sympathetic nervous system inhibits the parasympathetic activity while sympathetic stimulation of the α-adrenergic receptors in the internal sphincter muscle causes constriction and a rise in urethral pressure.13,48 During the filling phase the bladder is inactive until it holds approximately 350 to 500 mL of urine, even though a first sensation of filling may occur with 150 to 250 mL of urine in the bladder.77 When the bladder is full the receptors send a signal to the cortical centers of the brain, and as a result a voluntary micturition reflex is initiated to empty the bladder (see Figure 29-3, B).13,48,77 Involuntary contractions of the detrusor during the filling phase cause frequency and urgency (OAB syndrome). In neurological patients this is called hyperreflexia; if idiopathic, it is considered bladder instability.47,48 Individuals with a hypersensitive bladder or painful bladder syndrome (interstitial cystitis) empty their bladders frequently to avoid pain, which results in a functionally small bladder. When the sensation of bladder filling is decreased or absent, the bladder will overfill and urine can back up into the kidneys, causing dysfunction. Symptoms of overflow from the overstretched bladder frequently cause dribbling. All these problems require thorough medical investigation of possible causes. Preconditions for a normal storage phase as described by Henscher78 include good distensibility of the bladder, stable bladder without premature detrusor contractions (adequate bladder sensation, i.e., intact CNS and peripheral nervous system), no obstruction between kidney and bladder, and positive urethral closure at rest and under load. Micturition, or voiding, depends on the coordinated activity of the urethra and the detrusor muscle. The pelvic floor (levator ani and external sphincter muscles) has to relax when the detrusor muscle contracts. This occurs with activation of the parasympathetic cholinergic receptors in the bladder muscle. An afferent stimulus from the pelvic nerve (see Figure 29-3, A) reaches the PMC by way of the spinal cord. Efferent tracts inhibit the activity of the pudendal nerve, which results in relaxation of the external sphincter and levator ani. At the same time, sympathetic activity at the bladder neck is inhibited and postganglionic parasympathetic neurotransmitters are stimulated. This results in detrusor contraction.13,19,47,77 From the PMC, signals are also sent to the cerebral cortex, which allows voluntary control. An individual therefore can override the signal to empty the bladder and wait to empty it later or can empty the bladder when there is no signal that it is full. Suprapontine control of bladder function is a result of the modulating control of the brain stem, hypothalamus, and cerebral cortex.1,77 Many reflexes are involved in urine storage and voiding at various levels. The sacral reflex, for example, can be elicited by light stroking at the lateral aspect of the anus and should result in a symmetrical contraction of the anal sphincter (“anal wink”). Absence of the anal wink can be an indication of a neurological problem at S2 to S4, resulting in weakness or paralysis of the pudendal nerve.13 The micturition reflex, on the other hand, depends on an intact PMC in the brain stem.77 Prerequisites for a normal emptying phase include intact neural control, adequate functioning of the detrusor, no increase in resistance to voiding by obstruction, and adequate pelvic floor relaxation.78
Pelvic floor treatment of incontinence and other urinary dysfunctions in men and women
Overview of the clinical problem
History of pelvic floor exercises
Prevalence of urinary incontinence
Cost of incontinence
 Introduction of more continence-related products to the marketplace
Introduction of more continence-related products to the marketplace
 Change in the age composition of the U.S. population
Change in the age composition of the U.S. population
Definition of incontinence
Etiology
Anatomy and physiology of the pelvic floor
Endopelvic fascia
Pelvic diaphragm
Perineal membrane
Mucosal coaptation of the urethra
Internal sphincter of the urethra
Voiding mechanism of the bladder
Sympathetic innervation
Parasympathetic innervation
Somatic innervation
Sensory innervation
Filling and storage phase
Emptying phase
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree