Group
Bladder weight (g)
Sectional area of muscle fibers (μm2)
Percentage of connective tissue (%)
α-SMA activity (IOD)
A
0.138 ± 0.019
7,265.86 ± 114.36
12.08 ± 1.45
0.96 ± 0.03
B
0.286 ± 0.025
5,156.31 ± 310.42
18.47 ± 1.32
0.85 ± 0.10
C
0.362 ± 0.018
5,398.47 ± 453.08
23.24 ± 2.76
0.74 ± 0.06
D
0.412 ± 0.082
4,146.49 ± 284.06
26.29 ± 1.88
0.69 ± 0.09
E
0.496 ± 0.022a,b
3,067.40 ± 130.91a,b
35.67 ± 1.12a,b
0.44 ± 0.08a
F
0.589 ± 0.023a
2,123.56 ± 235.66a
47.22 ± 2.38a,b
0.39 ± 0.10a
G
0.721 ± 0.020a
1,675.98 ± 322.09a
55.56 ± 3.12a
0.36 ± 0.07a
4.3.1 Sectional Areas of DM Fibers
In group A, the DM fibers were in fusiform shape and funiculose; the cellular nucleus had consistent contours; and no infiltration of connective tissue could be detected in the muscular bundles. However, from groups B–G, the muscular fibers were gradually more crescent-shaped or irregular; they showed misalignment and disarray; and marked infiltration of connective tissue was observed in the muscle bundles (Fig. 4.1). Gradually progressive atrophy of the DM over time was found in groups B–G (Fig. 4.2). The sectional areas of the DM fibers in groups A–G were 7265.86 ± 114.36, 5156.31 ± 310.42, 5398.47 ± 453.08, 4146.49 ± 284.06, 3067.40 ± 130.91, 2123.56 ± 235.66, and 1675.98 ± 322.09 μm2, respectively. The sectional areas of muscle fibers in groups E, F, and G were significantly lower (P < 0.05) than that in control group A, but there was no significant difference between groups B, C, and D, and group A.
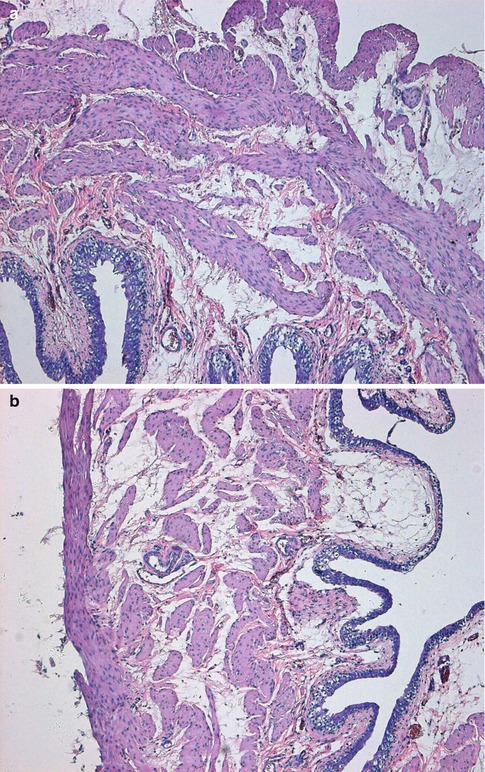
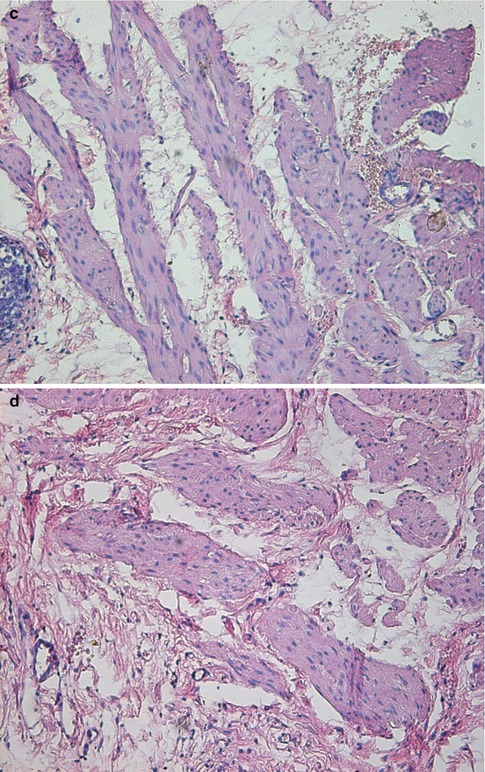
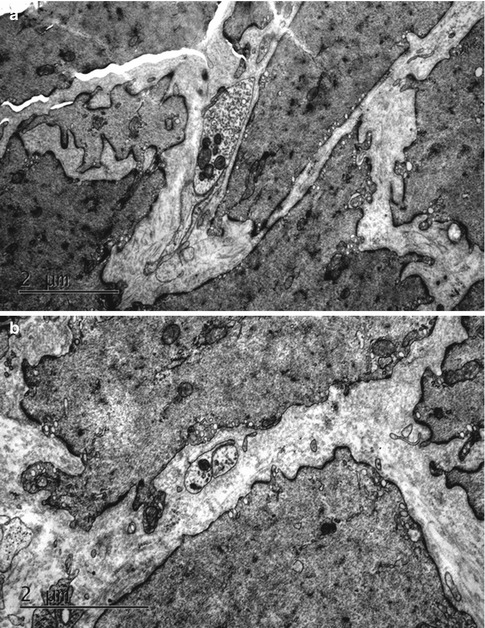
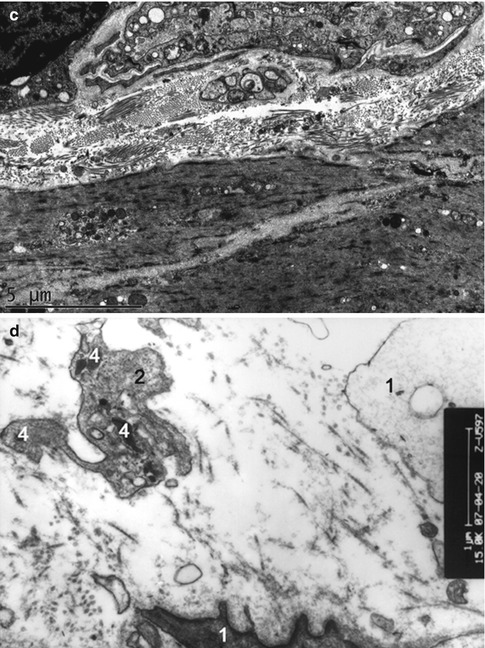


Fig. 4.1
Transverse sections of the detrusor muscle in rats of the normal control (a), and 6 weeks (b), 10 weeks (c) and 16 weeks (d) after medullary cone injury groups. Hematoxylin and eosin staining (×100)


Fig. 4.2
Ultrastructures of the detrusor muscle and its neuromuscular junction observed under a transmission electron microscope in rats of the normal control (a), and 6 weeks (b), 10 weeks (c) and 16 weeks (d) after medullary cone injury groups. 1 detrusor muscle, 2 neuromuscular junction, 3 synaptic vesicles, 4 degenerative corpuscle (×15,000)
4.3.2 Ultrastructure of the DM
In group A, DM cells were well-arranged and well-distributed with consistent contours and size, and normal intermediate junctions were observed, with the distance between muscular cells being much smaller than that in abnormal specimens. A few collagen fibers and elastic fibers could be seen in the matrix. Organelles such as the mitochondria and endocytoplasmic reticulum, myofilaments and dense bodies were well-organized in the smooth muscle cells. By contrast, in groups B–G, the atonic bladders showed abnormalities in the ultrastructure of detrusor cells that were gradually aggravated over time, such as inconsistent contours, misalignment and disarray; wide separation between muscular cells; abundant collagen fibers and irregular dense structures between individual cells; obviously widened rough endoplasmic reticulum (ER) and mitochondrial edema (Fig. 4.3).
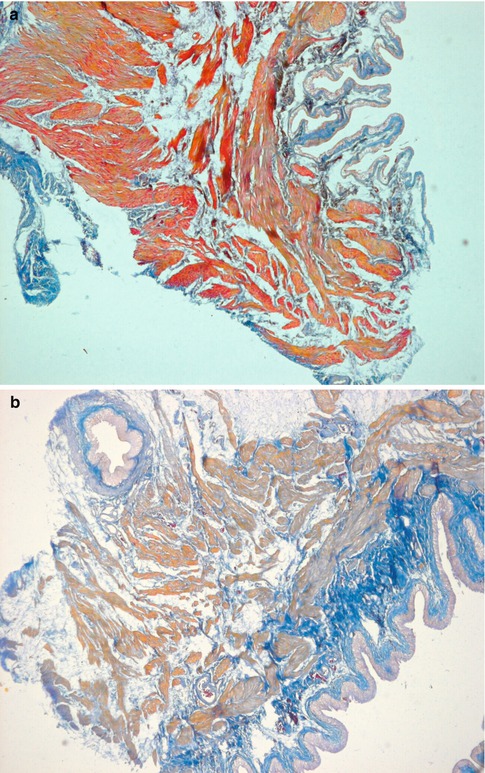
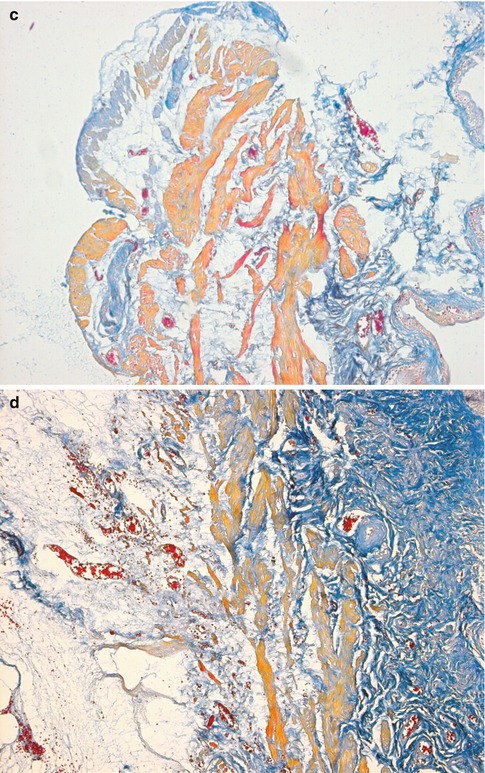


Fig. 4.3
Transverse sections of the detrusor muscle in rats of the normal control (a), and 6 weeks (b), 10 weeks (c) and 16 weeks (d) after medullary cone injury groups. Masson trichrome staining for analysis of fibrosis (×100)
4.3.3 Ultrastructure of the NMJ
In group A, the normal NMJ contained a large amount of organelles, such as mitochondria and ER, and abundant synaptic vesicles in the sympathetic nerve endings. The NMJ in groups B, C and D showed a lower amount of mitochondria and synaptic vesicles. Conspicuous degeneration of the NMJ was observed in group E; it was manifested as derangement or disappearance of the reductus, and obvious reduction in the amount of synaptic vesicles, mitochondria and ER in the sympathetic nerve endings. Further deterioration and obvious reduction or disappearance of synaptic vesicles and mitochondria, as well as degenerative corpuscles, was noted in group F. Finally, in group G, severe deterioration of the NMJ accompanied by absence of synaptic vesicles and a high amount of degenerative corpuscles were observed (Fig. 4.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








