Fig. 7.1
Illustration of the tendon-spinal cord-bladder artificial reflex arc in canines
7.2.2.2 Electrophysiologic Examination
The dogs were examined under anesthesia at the set postoperative times (6 months and 18 months). In the case of dogs 1–4, the skin was incised along the original incision. The scar outside the dura was stripped off. The dura was incised to separate the cauda equina carefully. The anastomosis was identified by means of the silk thread that was used as a label. For the experimental measurements, the recording electrode was inserted at the distal end of the anastomosis in the right S2 anterior motor root. Meanwhile, the stimulation electrode was inserted at the right L5 posterior sensory root. Both electrodes were connected to the EDJ-V Biosignal system (Shanghai Teaching Equipment Co., Shanghai, China), and unidirectional rectangular pulses of 115 mV were delivered for 1.0 ms. Then, action potential (AP) curves were recorded. For the control measurements, the recording electrode and the stimulation electrode were placed at the left S2 anterior motor root and the left S2 posterior sensory root respectively. The stimulation parameters were the same as those for the experimental measurements.
In the case of dogs 5 and 6, a median incision was made in the lower abdomen. For experimental measurements, the electrodes were inserted into the upper anterior wall of the bladder detrusor muscle, in order to stimulate the right L5 posterior sensory root, the right femoral nerve, and the afferent nerve of the knee-tendon reflex. The electrodes were connected to the Cantata 2000 muscular electrograph (Dantec Medical, Copenhagen, Denmark), and pulses of 3.8 mA were delivered at 1.0 Hz. Electromyograms (EMGs) of the detrusor muscle were recorded. For control measurements, the left S2 posterior sensory root was stimulated with the same parameters as those for the experimental measurements.
In both groups of dogs, paraplegia was induced by transecting the spinal cord acutely at the T10 canal level. Observations were carried out 48 h after paraplegia.
7.2.2.3 Measurement of Intravesical Pressure
As mentioned before, 48 h after the paraplegia model was induced, the dogs were observed under anesthesia. A median incision was made in the lower abdomen to unveil the bladder cervix and posterior urethra after anesthesia. A 12-F urinary catheter was inserted into the bladder through a small incision on the posterior urethra, after which the incision was sutured. The urinary catheter was then linked to a self-designed urodynamic 3-lead simplified pressure machine [8]. Through the fluid inlet, it was approximately 150 mL of normal saline injected into the bladder. In order to facilitate observing the results, the water column of the pressure test tube was maintained in the baseline of 10-cm. The fluid inlet was turned off. After this, the cauda equina was exposed via the original median approach to look for the previous anastomosis of two anterior motor roots (i.e. the right L5 and S2) in all dog samples. In the cases of Dogs 5 and 6, a right femoral anterior lateral incision was conducted to expose the femoral nerve acted as the afferent nerve of the knee-tendon reflex.
A series of stimulation currents of 1,000 mV at 10 Hz were delivered for 2 s from an EJD-V Biosignal system, in order to stimulate the proximal end of the right L5 anterior motor root anastomosis, right L5 posterior sensory root, left S2 anterior motor root, left L5 posterior sensory root, and right femoral nerve. A medical percussion hammer was used to percuss the right knee tendon and the left knee tendon for 10 s at a frequency of 2 times per second. Increase in bladder pressure (cm H2O) and duration of urination (T) were recorded. The left S2 anterior motor root served as the normal control, while the left L5 posterior sensory root and left knee tendon were utilized as negative controls.
7.2.2.4 Neurohistologic Observations
A nerve specimen was derived from the region 0.5 cm proximal and 1.0 cm distal to the anastomosis, and it was stained with HE in order to count the number of fibers containing medullary nerves with the Leica FW4000 Image Analysis System (Leica, Solms, Germany). The passing rate of myelinic nerve fibers was determined by dividing the number of fibers at the distal end with that at the proximal end.
Data were analyzed using SPSS12.0 and expressed as mean ± SD. The Student’s t-test was used to analyze data. P < 0.05 was considered to indicate statistical significance.
7.2.3 Results
7.2.3.1 Electrophysiologic Examination
The same current was released to stimulate L5 posterior sensory nerve root before paraplegia, which was repeated 48 h after complete paraplegia at the T10 canal level. In all 6 dogs, AP curves were found in S2 anterior motor root distal to the anastomosis. The morphology and amplitude of these curves were similar to those of the control side (Fig. 7.2). After the 48 h of complete paraplegia at the T10 canal level, EMG of the detrusor muscle was found in both the right L5 posterior sensory root and right femoral nerve. They shared similarity in both morphology and amplitude with those of the left S2 posterior sensory root of the control, whereas stimulation of the left L5 posterior sensory root failed to trigger an EMG response of the detrusor muscle (Fig. 7.3).
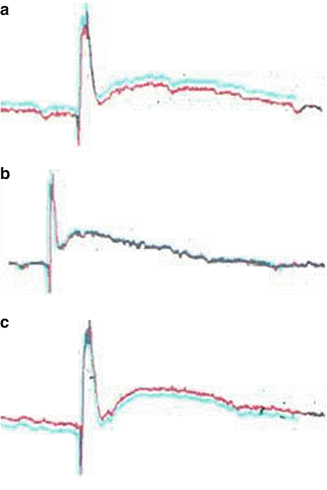
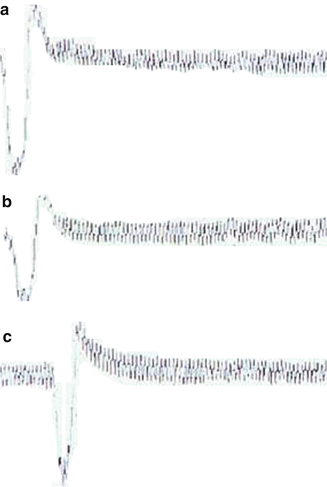

Fig. 7.2
AP curves of dogs 5 (left) and 6 (right), which were obtained by stimulation at the left S2 anterior motor root and recording at the left S2 posterior sensory root (a). The right L5 posterior sensory root was stimulated before (b) and 48 h after complete paraplegia (c), with the nerve recordings taken at the distal ends of the anastomosis

Fig. 7.3
EMGs of the detrusor muscle of dogs 5 (left) and 6 (right), which were obtained by stimulation at the left S2 posterior sensory root (a), right L5 posterior sensory root (b), and right femoral nerve (c), which was the afferent nerve of the knee-tendon reflex
7.2.3.2 Intravesical Pressure Measurement
In dogs 1–4, mean bladder contraction and mean duration of the urination were 59 % ± 23 % and 83 % ± 31 % that of the control group; mean bladder contraction and mean duration induced by percussion of the right knee tendon were 38 % ± 27 % and 51 % ± 37 % that of the control group respectively. In dogs 5 and 6, mean bladder contraction and mean duration were 84 % ± 5 % and 88 % ± 6 % that of the control group; the values obtained on percussion of the right knee tendon were 62 % ± 5 % and 84 % ± 12 % that of the control group. Thus, compared with the 6-month group, the 18-month group experienced a significant increase in bladder pressure (P < 0.05). Moreover, the duration of urination was also significantly longer in the 18-month group (P < 0.05). However, no significant different in duration between the groups was observed with percussion of the right knee tendon (P > 0.05).
7.2.3.3 Neurohistologic Observations
HE staining unveiled that numerous nerve fiber growths passed through the anastomoses on the experimental side. The longitudinal section had regenerated nerve fibers that were well arranged and grew in the same direction. The cross-sectional view showed regenerated nerve fibers that presented with a typical myelinated nerve fiber structure (i.e., myelin sheath at the periphery and nerve axon in the center). The figures for nerve fibers distal and proximal to the anastomosis on the experimental side were 574 ± 261 and 988 ± 124 respectively. The passing rate of myelinated nerve fibers was in the range of 58.1 % ± 13.4 %. By contrast, the figure for fibers of the left S2 anterior root was around 867 ± 349. The difference between the two sides was statistically significant (P < 0.05).
7.2.4 Discussion
The purpose of this study was to explore the potential effects of creating a new knee tendon-bladder reflex arc below the paraplegic level in dogs that had sustained SCI above the medullary cone. A combination of electrophysiological and neurohistological methods were used to study the new reflex arc established. Specifically, electrical stimulation in the six dogs showed that the new reflex arc was able to induce bladder contraction; in two dogs however, bladder contraction was also induced by percussion of the knee-tendon. This can be attributed to poor quality of the anastomosis without enough axons passing through the stoma in a timely fashion. It may have also resulted from mismatch of the nerve fibers.
We found that bladder contraction in the 18th month was significantly stronger than that in the 6th month after surgery; this indicated that long-term axon regeneration, reinstitution of the bladder nerve, and recovery of bladder function were better than those in the early stages after the operation.
Our neurohistologic results showed that numerous nerve fiber growths passed through the anastomosis at the experimental sides. This also indicated that the autonomic nerve fibers of S2 can be reinnervated by the somatic nerve fibers of L5. Therefore, reconstructed bladder reinnervation below the level of SCI could facilitate urination via the knee tendon. Moreover, the motor impulses of the somatic reflex caused by percussion of the knee tendon were transmitted to the bladder through the motor efferent branch, leading to spontaneous contraction of the bladder.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








