Partial Rotator Cuff Tears: Treatment Options
William B. Stetson
Partial thickness tears of the rotator cuff may involve either the articular surface, bursal surface or both sides of the rotator cuff or can be intratendinous. They may be asymptomatic or a potential source of shoulder dysfunction. With the advent of magnetic resonance imaging (MRI) and shoulder arthroscopy, more tears are being recognized. However, the optimal clinical approach to these tears has not been completely defined (1). To gain a better understanding of these tears, we must first understand the anatomy, pathogenesis, and natural history of these tears and then agree on a classification system to develop a rational approach to their treatment.
ANATOMY
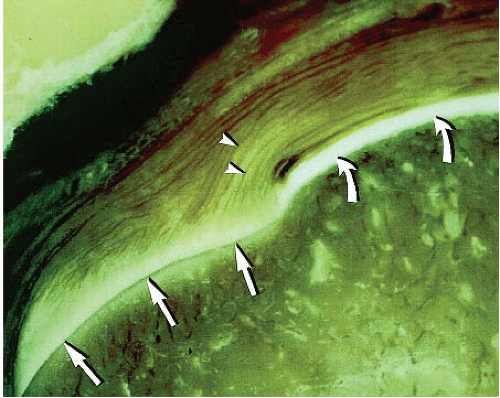
The suprascapular artery is the primary vascular supply to the supraspinatus tendon. The vascular studies of Rathbun and McNab have demonstrated the articular side of the rotator cuff is hypovascular as compared to the bursal side. This finding has been suggested as a factor in the tendency for partial tears to occur on the articular surface of the cuff (Fig. 8.1). Perfusion of the rotator cuff is a dynamic phenomenon with markedly reduced perfusion when the arm is in full adduction. Collagen bundles located near the articular surface of the cuff are thinner and less uniform than the thick parallel bundles found closer to the bursal surface (Fig. 8.2). The articular surface of the cuff has an ultimate failing stress only half as high as the bursal surface. This lack of uniformity of the collagen bundles along with the hypovascularity of the articular surface of the cuff are contributing factors for partial tears to occur more commonly on the articular surface (1).
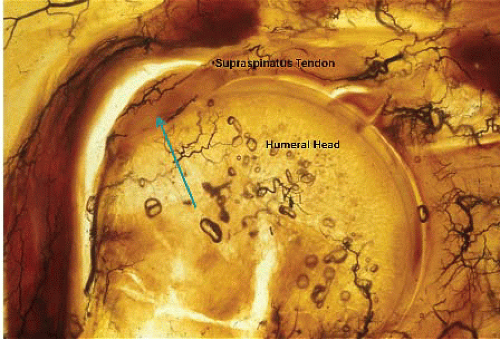 FIGURE 8.1. Coronal photomicrograph of the zone of diminished vascularity of the supraspinatus tendon. The arrow points to the critical zone of hypovascularity of the articular side of the tendon. |
The anatomic footprint of the rotator cuff has been proposed as an important landmark for the insertional point of the supraspinatus tendon and for recognizing the degree of partial tearing of the articular surface of the rotator cuff. In a cadaveric study, Curtis studied the anatomic insertions of the rotator cuff musculature. He found the supraspinatus had a rectangular insertion from approximately the 11:30 position to the 1 o’clock position with an average length of 23 mm (range 18 to 33 mm) and a width of 16 mm (range 12 to 21 mm) (Fig. 8.3A, B). The infraspinatus wraps and interdigitates with the supraspinatus tendon. The infraspinatus frames the bare spot of
the humeral head, has an average length of 28 mm (range 20 to 45 mm) and a width of 18 mm (range 12 to 24 mm) (2). Nottage and his colleagues found the mean anteroposterior dimension of the supraspinatus tendon was 25 mm. The mean superior to inferior tendon thickness at the rotator interval was 11.6 mm, 12.1 mm at mid-tendon, and 12 mm at the posterior edge (3). Mochizuki and colleagues studied the humeral insertions of the supraspinatus and infraspinatus tendons in 113 cadaver shoulders. The footprint of the supraspinatus was triangular in shape with an average medial to lateral length of the tendon to be 6.9 mm (±1.4 mm). The average anterior to posterior width was 12.6 mm (±2.0 mm) on the medial margin and 1.3 mm (±1.4 mm) on the lateral margin. The infraspinatus had a long tendinous portion in the superior half of the muscle, which curved anteriorly and extended to the anterolateral area of the highest impression of the greater tuberosity. The footprint of the infraspinatus was trapezoidal in shape with an average medial to lateral length of 10.2 mm and an average anterior-posterior width of 32.7 mm. They found that the footprint of the supraspinatus on the greater tuberosity was smaller than previously believed and that this area of the greater tuberosity is actually occupied by a substantial amount of the infraspinatus. The normal cuff is 9 to 12 mm thick but ranges from 9 to 22 mm of thickness.
the humeral head, has an average length of 28 mm (range 20 to 45 mm) and a width of 18 mm (range 12 to 24 mm) (2). Nottage and his colleagues found the mean anteroposterior dimension of the supraspinatus tendon was 25 mm. The mean superior to inferior tendon thickness at the rotator interval was 11.6 mm, 12.1 mm at mid-tendon, and 12 mm at the posterior edge (3). Mochizuki and colleagues studied the humeral insertions of the supraspinatus and infraspinatus tendons in 113 cadaver shoulders. The footprint of the supraspinatus was triangular in shape with an average medial to lateral length of the tendon to be 6.9 mm (±1.4 mm). The average anterior to posterior width was 12.6 mm (±2.0 mm) on the medial margin and 1.3 mm (±1.4 mm) on the lateral margin. The infraspinatus had a long tendinous portion in the superior half of the muscle, which curved anteriorly and extended to the anterolateral area of the highest impression of the greater tuberosity. The footprint of the infraspinatus was trapezoidal in shape with an average medial to lateral length of 10.2 mm and an average anterior-posterior width of 32.7 mm. They found that the footprint of the supraspinatus on the greater tuberosity was smaller than previously believed and that this area of the greater tuberosity is actually occupied by a substantial amount of the infraspinatus. The normal cuff is 9 to 12 mm thick but ranges from 9 to 22 mm of thickness.
PATHOGENESIS
The pathogenesis of partial rotator cuff tears are multifactorial and may be classified as intrinsic, extrinsic, traumatic or a combination of all of these. Intrinsic changes in the cuff are related to intrinsic tendinopathy with failure of collagen fibers within the cuff. This may be due to a lack of uniformity of the collagen bundles especially on the articular side causing partial tearing on the articular side. The lack of cuff vascularity also contributes to weakness of the cuff on the articular side leading to degenerative tears associated with the aging process. These degenerative tears are often associated with extensive delamination or can remain entirely intratendinous.
Histologic evidence supporting an intrinsic, degenerative cause of rotator cuff tears with aging by some researchers has shown a loss of cellularity, loss of vascularity, and loss of fibrocartilage mass at the site of the cuff insertion. Hashimoto and colleagues found seven characteristic features of age-related degeneration in tissue specimens which included thinning and disorientation of the collagen fibers, myxoid degeneration, and hyaline degeneration. Other degenerative changes included vascular proliferation, fatty infiltration, chondroid metaplasia and calcification. The authors felt that vascular proliferation was part of the reparative process. Histologic evidence from surgical specimens does not support a significant reparative process nor any significant inflammatory process. Although inflammation may play a role in the initiation of rotator cuff pathology, it appears not to play a role in the propagation and progression of the disease process. These specimens typically showed hypercellularity, a loss of tightly bundled collagen appearance, an increase in proteoglycan content, and neovascualization. This is felt to be a failed healing response. Therefore, the term tendinopathy may be more appropriate than tendonitis or tendinosis as a generic descriptor of the clinical condition seen in the shoulder.
Smooth muscle actin (SMA) has been found within the nonvascular connective tissue cells immediately surrounding torn rotator cuff edges. SMA in vitro leads to contraction of collagen-glysosaminoglycan compounds, substances found in considerable concentrations in the rotator cuff. In vivo, this may translate into SMA cells
causing the damaged cuff to retract with the increasing distances at the repair margin, which results in an inhibition of healing.
causing the damaged cuff to retract with the increasing distances at the repair margin, which results in an inhibition of healing.
The role of altered collagen fiber quality has also been proposed as an intrinsic mediator of cuff degeneration. The healthy central zone of the supraspinatus tendon is primarily composed of type I collagen with smaller amounts of type III collagen. The fibrocartilaginous zone of the tendon insertion against the humerus is primarily composed of type II collagen, a collagen subtype associated with withstanding compressive loads. In diseased rotator cuff there is an increase in the levels of type III collagen within the fibrocartilaginous zone, a collagen subtype associated with tendon healing and a decrease in type II collagen. The change in collagen composition could reduce the tendon’s ability to withstand the compressive loads traditionally associated with type II collagen.
Extrinsic impingement due to narrowing of the supraspinatus outlet caused by coracoacromial arch abnormalities can result in cuff irritation and may play a major role in many partial cuff tears (1). Histologic changes have been found on the undersurface of cadaveric acromion specimens with bursal surface tears but not in those with articular surface tears. This suggests that bursal-surface tears may be more likely to be related to abrasion of the cuff by the acromion (1). Gartsman and Milne (4) believe that extrinsic impingement due to coracoacromial arch narrowing can lead to partial tears on the articular side as well as the bursal surface of the cuff. A differential shear stress affecting the layered anatomy of the cuff has been proposed as another mechanism involved in the production of articular surface tears (1).
Walch et al. (5) and Jobe have described a subset of partial articular-sided rotator cuff tears that develop secondary to “internal impingement.” Glenohumeral instability and traction stress on the rotator cuff in the throwing athlete can lead to undersurface tears in the absence of extrinsic impingement. In addition, throwers and other overhand athletes may experience posterior shoulder pain when repetitive contact occurs between the undersurface of the supraspinatus and the posterosuperior glenoid during the late cocking phase of the throwing motion. Fatigue of the dynamic stabilizers and excessive external rotation secondary to overstretching of the anterior capsule may predispose individuals to development of internal impingement (1). A subset of these patients may develop a glenohumeral internal rotation deficit (GIRD) with a significant loss of internal rotation on the affected side.
Trauma is more often associated with articular surface tears than with bursal surface tears (6). This may be due to a direct fall on the shoulder or to repetitive microtrauma seen in laborers or athletes with repetitive overhead activities. Repetitive stresses may lead to small injuries within the tendon that are given an insufficient time to heal before further trauma. The combination of weaker cuffs with a single traumatic insult, or progressive microtrauma can then lead to cuff tearing. This is consistent with the early studies of Codman that demonstrated that partial cuff tears typically began on the articular side of the tendon, because the load capacity of the bursal side is higher than that of the articular side, making the articular side more prone to damage. Typically, after the deep fibers tear, they retract because they remain under tension even with the arm at rest. This results in an increased load on the remaining fibers that increases the likelihood of further rupture.
Repetitive trauma and chronic overuse have been studied in the rat model and angiogenic and inflammatory markers have been identified and support the hypothesis that both of these play an important role in tendon degeneration. Substance P, a proinflammatory mediator, has been found to be increased in rotator cuff tendinopathy. Acute increases in angiogenic messenger ribonucleic acid (mRNA) markers for vascular endothelial growth factor (VEGF) have been found in the cuff of rats undergoing repetitive microtrauma injuries. Overuse also leads to a progressive down regulation of transforming growth factor beta-1 (TGF-β1), altering the normal collagen constituents within the rat supraspinatus tendon and leading to a lower load to failure with respect to controls. Overload not only affects the collagen and proteoglycans but also elicits an essential response in tenocytes that appears designed to adapt the collagen matrix to increased load. Matrix load is transmitted into the cell and alters protein and enzyme production and can actually cause nucleus deformation. Clearly, this is one of the most intriguing and dynamic fields of research.
The current human in vivo evidence base for a strong inflammatory component remains relatively weak as histologic studies have failed to find a significant chronic inflammatory environment in rotator cuff tears and other tendinopathies in cadaveric and postsurgical specimens.
In summary, degeneration and partial tearing of the rotator cuff tendon is multifaceted. A combination of growth factors and neurotransmitters affects the tenocytes, their nuclei and the collagen infrastructure of the rotator cuff tendon. On a histologic level, degeneration is characterized by loss of cellularity, vascularity, tissue architecture, and fibrocartilaginous mass within the cuff tendon resulting in a mechanically inferior tendon. This is then compounded by repetitive microtrauma—mechanical loading of a degenerative tendon leading to several small tears that only partially heal until the tendon is so weakened that a full-thickness tear develops.
NATURAL HISTORY
The natural history and progression of these partial rotator cuff tears is a controversial topic. Codman first described changes in the musculotendinous cuff as a rim rent (partial tear) in the undersurface of the supraspinatus tendon at the point of attachment near the articular surface of the
humeral head (7). We do know that partial tears of the articular surface are two to three times more common than bursal surface tears (8, 9). Most tears involve the supraspinatus tendon with the infraspinatus, subscapularis, and teres minor tendons much less commonly involved (4). Intratendinous tears are intrasubstance and therefore have no communication with either surface (10). As anticipated, cadaveric studies have demonstrated a higher incidence of intratendinous tears than that reported in clinical studies since inspection is limited to the tendon surfaces (11). MRI techniques have improved our ability to detect intrasubstance tears and tendon degeneration.
humeral head (7). We do know that partial tears of the articular surface are two to three times more common than bursal surface tears (8, 9). Most tears involve the supraspinatus tendon with the infraspinatus, subscapularis, and teres minor tendons much less commonly involved (4). Intratendinous tears are intrasubstance and therefore have no communication with either surface (10). As anticipated, cadaveric studies have demonstrated a higher incidence of intratendinous tears than that reported in clinical studies since inspection is limited to the tendon surfaces (11). MRI techniques have improved our ability to detect intrasubstance tears and tendon degeneration.
Fukuda et al. (11) reported a 13% incidence of partial rotator cuff tears in a cadaveric study of 249 anatomic specimens. The prevalence of these partial thickness tears increases with age. DePalma studied 96 shoulders of patients aged 18 to 74 years without a history of shoulder dysfunction and found partial ruptures of the supraspinatus tendon in 37%.
Sher and colleagues (12) in an MRI study of 96 asymptomatic individuals, found a high incidence of partial rotator cuff tears. They were increasingly frequent with advancing age and were compatible with normal, painless, functional activity.
In 1934, Codman (7) described four categories of incomplete rupture of the rotator cuff. He suggested that spontaneous healing of partial thickness rotator cuff tears might occur. In 1996, Fukuda et al. (10) examined histologic sections of partial thickness rotator cuff tears and found no evidence of active tissue repair. The question of whether or not these partial tears heal or progress is controversial. However, second look arthroscopies of patients with documented partial rotator cuff tears have demonstrated no evidence of healing (4, 9). Yamanaka studied 40 patients with symptomatic articular-sided partial rotator cuff tears treated nonoperatively with serial arthrography. At a mean follow-up of 13.5 months, repeat arthrography showed that four tears (10%) had disappeared and were presumed to have healed, reduction of the size of the tear occurred in 4 patients (10%), but enlargement of tear size occurred in 79% with 21(51%) of the partial tears increasing in size, and 11 (28%) progressed to a full thickness tear. Mazoue and Andrews followed 11 baseball pitchers who had arthroscopic debridement of partial rotator cuff tears but were unable to return to pitching. At the time of repeat arthroscopy, 9 progressed to a full thickness tear. With repetitive microtrauma seen in overhead athletes such as pitchers, the risk of progression to a full thickness tear can be as high as 81% (13).
The prognosis of articular-sided tears appears to be worse with increasing age, a larger initial tear size, and the absence of a traumatic episode. The patients in Yamanaka’s study in whom follow-up arthrography showed a disappearance of the tear often had a history of trauma. Conversely, a history of trauma was seldom noted in those with progressive tear enlargement, resulting in a full thickness tear. Therefore, the risk of progression of partial thickness tears to a full thickness tear is significant and ranges from 28% to 81% (13).
A recent MRI study published in 2009 of partial thickness rotator cuff tears treated nonoperatively showed a progression of the tear in only 17%. However, this may be explained by the use of different diagnostic techniques of arthrography in Yamanaka’s study compared with MRI in this recent study by Maman. Future studies using magnetic resonance arthrography (MRA) may be helpful in explaining this discrepancy as MRA has been found more sensitive in detecting partial articular-sided rotator cuff tears (14).
The role of operative treatment in modifying the natural history of partial thickness rotator cuff tears is poorly defined (1). Although Codman felt debridement of partial tears stimulated a healing response (7), there is no evidence that debridement of a partially torn cuff stimulates a healing response (4,9) in second look arthroscopies. The role of subacromial decompression and its ability to reduce narrowing of the subacromial outlet in those with external impingement has been proposed to delay the progression of cuff pathology but has never been proven in prospective, clinical studies.
The question of which partial tears may progress and why still needs to be clearly defined. At this point, it appears prudent to follow patients clinically and monitor their symptoms. If their symptoms progress or do not resolve after being treated with proper nonoperative means, the partial tear may be a cause of their symptoms or the tear may be progressing in size and may need to be addressed surgically. Overhead athletes may be more prone to progression of their partial thickness tears to complete tears because of the repetitive stresses placed on the rotator cuff (13).
CLINICAL EVALUATION
Pertinent History and Physical Examination
The prevalence of partial tears of the rotator cuff in asymptomatic individuals was studied by Sher et al. in 1995 (12). Magnetic resonance images of the shoulders of 96 asymptomatic individuals found 19 partial thickness tears (20%). It is evident from this study that partial tears may be asymptomatic and must be evaluated on a case-by-case basis to determine if the partial tear is truly causing symptoms.
It is important to take a detailed history of the patient starting with the duration of symptoms and the mechanism of injury. Patients may present with an insidious onset of shoulder pain without any discrete injury or accident. They may also have a history of trauma or repetitive stress to the shoulder as seen with overhead athletes. Pain is the predominant symptom, often most troubling at night and with overhead activities (11). Their symptoms may be nonspecific and may overlap with impingement, rotator cuff tendonitis or tendinopathy, and small, full thickness
rotator cuff tears (15). Most patients have a painful arc of motion between 60° and 120° of elevation (6). They may also have loss of motion (10) with posterior capsular tightness and a resultant restriction of internal rotation (4). This may cause anterosuperior translation of the humeral head from a posterior capsular contracture and may potentiate impingement-like symptoms (1).
rotator cuff tears (15). Most patients have a painful arc of motion between 60° and 120° of elevation (6). They may also have loss of motion (10) with posterior capsular tightness and a resultant restriction of internal rotation (4). This may cause anterosuperior translation of the humeral head from a posterior capsular contracture and may potentiate impingement-like symptoms (1).
The impingement signs described by Neer (pain with forced passive forward elevation) and Hawkins (pain with passive internal rotation of the arm placed in 90° of abduction) are positive in nearly all patients with symptomatic partial thickness rotator cuff tears (4). Differentiating between impingement alone and impingement with a partial rotator cuff tear can be very difficult. It is at times difficult to determine what is causing the pain. Is it more the impingement or is the pain caused more by the partial rotator cuff tear? The lidocaine test, an injection of 10 cc of 1% lidocaine into the subacromial space, can be helpful. After the injection, the maneuvers may be repeated and diminution of pain on repeat testing may be indicative of pure impingement (1).
Strength is usually preserved on clinical examination. However, pain inhibition may result in an apparent loss of strength and a decrease in active range of motion in these patients with a partially torn rotator cuff (1). Patients may also have pain with active resistance to shoulder abduction with the shoulder positioned in 90° of abduction in the scapular plane (Jobe test).
Throwing athletes with partial thickness rotator cuff tears may also have nonspecific posterior shoulder pain, indicative of “internal impingement.” They may develop a GIRD or contracture with an obligate increase in external rotation (5). Impingement of the deep surface of the supraspinatus tendon may occur as the cuff abrades against the posterosuperior glenoid rim (5). It is a matter of debate whether or not rotator cuff injuries observed in individuals with internal impingement develop as a result of pathologic anterior glenohumeral subluxation or repetitive cuff abrasion in an otherwise stable shoulder (1). Posterosuperior labral lesions (SLAP variants) may also be present in throwing athletes and predispose to articular surface partial thickness rotator cuff tears (5).
The clinical course of patients with partial thickness rotator cuff tears is often indistinguishable from that of patients with impingement syndrome, tendonitis or tendinopathy, or small, full thickness rotator cuff tears. Symptoms may also be difficult to differentiate from bicipital tendonitis, labral or SLAP lesions, and mild cases of adhesive capsulitis (1). These associated conditions may also be present in addition to rotator cuff pathology creating a confusing clinical presentation.
Imaging
Imaging techniques to detect partial thickness rotator cuff tears have improved over the last 10 years. With the advent of MRA and newer fat suppression techniques, sensitivity has increased in detecting partial tears.
Radiographic evaluation is the first imaging tool used when evaluating shoulder pathology. Initial X-rays include an anteroposterior view of the glenohumeral joint, an axillary view, and a supraspinatus outlet view. The supraspinatus outlet view is especially important because it not only demonstrates acromial morphology (types I to III), but it also ascertains the thickness of the acromion which is important in preoperative planning for an arthroscopic subacromial decompression. In general, radiographic findings are nonspecific for partial thickness tears but may be helpful in ruling out other causes of shoulder pain (1).
Arthrography of the glenohumeral joint is limited for detecting partial tears. Although proponents have reported an accuracy of >80% (6), other authors have been unable to duplicate these results (4, 5).
Bursography may be performed as an adjunct to arthrography to aid in the detection of partial thickness tears involving the bursal surface. However, subacromial inflammation and adhesive capsulitis may limit the value of this technique (1). The accuracy of bursography has been reported to range from 25% to 67% (6, 10, 11). However, a negative arthrogram or bursogram cannot reliably rule out the presence of a partial thickness rotator cuff tear (15).
Ultrasound may be of limited value in detecting partial thickness tears. Weiner and Seitz reported a sensitivity of 94% and a specificity of 93% in a series of 69 partial thickness rotator cuff tears. However, its clinical use is limited by the availability of personnel experienced in the performance and interpretation of the study and its limitations in diagnosing other concomitant pathology. In comparing preoperative ultrasound to MRI in arthroscopically confirmed partial-thickness rotator cuff tears, Teefey found that 13 of 19 tears were correctly identified by ultrasound, whereas 12 of 19 tears were correctly identified by MRI. Iannotti found the preoperative diagnostic accuracy of ultrasound to be 70% and MRI to be 73%.
MRI, although a useful and established technique for detecting full thickness rotator cuff tears, has been found to be less reliable in detecting partial tears. Newer techniques have been developed to increase sensitivity. Using a T1-weighted image, a diagnosis of a partial tear is suggested by increased signal in the rotator cuff without evidence of tendon discontinuity. Further increase in signal changes on a T2-weighted with a focal defect can suggest an intratendinous tear (Fig. 8.4A, B, C). Signal changes on the bursal side can be indicative of a bursal-sided tear, which can be accentuated by fluid within the subacromial space (Fig. 8.5). Signal changes on a T2-weighted image can also show an articular surface tear (Fig. 8.6). Rotator cuff tendonitis or tendinopathy may be distinguished from partial thickness tears by an increased signal on T1 images but a decreased signal on T2 (Fig. 8.7A, B). However, many cases of tendinopathy may actually be partial thickness rotator cuff tears (15).
Standard magnetic resonance techniques are relatively insensitive in the detection of partial thickness
tears. In 1992, Traughber and Goodwin reported a sensitivity of 56% to 72% and a specificity of 83% to 85% for arthroscopically proven partial thickness cuff tears. Other studies such as that by Hodler and Snyder in 1992 have reported an 83% rate of false negative results with arthroscopically proven partial tears. Wright and Cofield found only six definite partial tears on preoperative MRI studies in 18 patients with arthroscopically proven partial tears.
tears. In 1992, Traughber and Goodwin reported a sensitivity of 56% to 72% and a specificity of 83% to 85% for arthroscopically proven partial thickness cuff tears. Other studies such as that by Hodler and Snyder in 1992 have reported an 83% rate of false negative results with arthroscopically proven partial tears. Wright and Cofield found only six definite partial tears on preoperative MRI studies in 18 patients with arthroscopically proven partial tears.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree