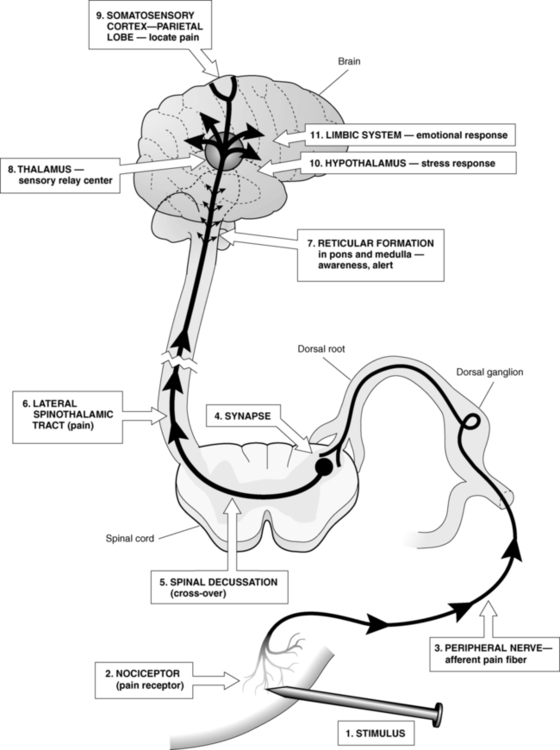
After reading this chapter the student or therapist will be able to: 1. Describe the pain pathways. 2. Describe how pain is modulated within the nervous system. 3. Identify the causes of acute and chronic pain. 4. List the signs and symptoms of CNS, ANS, and peripheral pain and give an example of each. 5. Perform a comprehensive pain evaluation, including taking a pain history, measuring pain intensity, measuring pain character, and examining the client. 6. Design a comprehensive pain management program that addresses the objective and subjective aspects of the pain experience. Chronic pain is prevalent. The National Institutes of Health (NIH) estimate that 100 million Americans suffer from chronic pain.1 The prevalence of chronic noncancer pain in patients seen in the primary care setting shows an approximate range of 5% to 33%,2 and a 2006 American Pain Foundation survey found that fewer than 40% of people with chronic noncancer pain reported that their pain was under control.3 Studies of physicians, nurses, and therapists who treat individuals with chronic pain show that most do not have even a basic understanding of the concepts of pain management.4–6 The result is inadequate or inappropriate care6–8 of individuals who report having pain. The use of the International Classification of Functioning, Disability and Health (ICF) model as a framework for understanding the relationship among impairments, activity limitations, and participation restrictions has been covered previously (refer to Chapter 1 as well as many chapters in the second section of this text). In terms of the application of the ICF model to pain, the clinician must understand that many impairments within various body systems cause pain and can limit activity and participation. The ability to identify appropriate rehabilitation approaches to improve activity and participation depends to a great extent on the clinician’s ability to identify different impairments that cause pain, or the resulting impairments and activity and participation problems caused by pain. The primary purpose of pain is to protect the body. It occurs whenever there is tissue damage, and it causes the individual to react to remove the painful stimulus. Pain is also a sensation with more than one dimension. To the individual, pain is both an objective and a subjective experience. The objective dimension is the physiological tissue damage causing the pain. The subjective dimensions include the following9: Pain arises from the stimulation of specialized peripheral free nerve endings called nociceptors. Injurious stimulation to the skin, muscle, joint, viscera, or tissue can trigger these peripheral terminals, whose cell bodies are located in the dorsal root ganglia and trigeminal ganglia. The density of nociceptors varies between as well as within these tissues. Nociceptors are extremely heterogeneous, differing in the neurotransmitters they contain, the receptors and ion channels they express, their speed of conduction, their response properties to noxious stimuli, and their capacity to be sensitized during inflammation, injury, and disease.10 Nociceptors found in interstitial tissues become excited with extreme mechanical, thermal, and chemical stimulation,11 whereas nociceptors found in vessel walls become excited with these stimuli plus marked constriction and dilation of the vessels.12 These receptors respond directly to some noxious stimuli and indirectly to others by means of one or more chemicals (histamine, potassium, bradykinin) released from cells in the traumatized tissues.13 These three types of nociceptors are broadly distributed in the skin and tissues and may work together. One example would be hitting one’s shin against a table: a sharp “first pain” is felt immediately, followed later by a more prolonged aching, sometimes burning, “second pain.”13 The fast, sharp pain is transmitted by A delta fibers that carry information from thermal and mechanical nociceptors. The slow, dull pain is transmitted by C fibers that are activated by polymodal nociceptors. Nociceptive input travels on A delta and C fibers into the dorsal horn of the spinal cord, where the gray matter is laminated and organized by cytological features. The first-order A delta and C fibers synapse with second-order neurons in lamina I (marginal layer), II (the substantia gelatinosa [SG]), and V. The second-order neurons do one of three things. A small number synapse with motor neurons, causing reflex movements (e.g., withdrawing the hand from a hot object). Others synapse with autonomic fibers, causing responses such as changes in heart rate and blood pressure and localized vasodilation, piloerection, and sweating. Most, however, travel a multisynaptic route to the higher centers by means of the ascending tracts.11,14 There are four major classes of neurons15 responding to pain in the dorsal horn: low-threshold nociceptive-specific neurons designated class I; wide dynamic range (WDR) neurons designated class II; high-threshold nociceptive neurons designated class III; and a fourth, nonresponder group of neurons that develop spontaneous activity with exposure to endogenous inflammatory cytokines, designated class IV. Nociceptive-specific neurons are most abundant in superficial lamina; their receptor fields are discrete and vary from one to several square centimeters.16 WDR neurons, in contrast, respond to a wide range of stimuli from A delta, A beta, and C fibers in a graded manner (i.e., the rate of firing escalates with increasing intensity of stimulation), can be found in all lamina, and are the most prevalent cells in the dorsal horn.16 Because of their unique response to innocuous or nociceptive input, as well as their larger receptor field, WDR neurons play an important role in the central sensitization and the plasticity of the spinal cord.16 Nociceptive input crosses at the cord level to the anterolateral quadrant of the ascending contralateral spinothalamic tract (Figure 32-1). The axons of the anterolateral quadrant are arranged so that the sacral segments are most lateral, with the lumbar segments more medial and the cervical segments most central. This arrangement may be important clinically in that symptoms may be provoked according to dermatomal maps to some degree.17 Pain dermatomes overlap to several adjacent dorsal roots so boundaries can be less distinct, requiring the clinician to distinguish the pain and dysfunction. The thalamus processes and relays information to several higher centers.12 Each projection serves a specific purpose. Axons of the spinothalamic tract project information to both the lateral and medial nuclear groups of the thalamus. The lateral nuclear group of the thalamus is where information about the location of an injury is thought to be mediated.13 Injury to the spinothalamic tract and the lateral nuclear group of the thalamus causes central neuropathic pain, which is discussed in further detail later. Ascending transmission of pain impulses is mediated by the action of the chemical excitatory neurotransmitter glutamate (A delta and C fibers) and tachykinins such as substance P (C fibers). Glutamate and neuropeptides have distinct actions on postsynaptic neurons, but they act together to regulate the firing properties postsynaptically.13 Tachykinins’ activity is thought to prolong the action of glutamate, as levels are increased in persistent pain conditions.16 The substrates of nociception that exist at the spinal level are complex in that more than 30 different neurotransmitters acting on more than 50 different receptors have been identified in the spinal cord and associated with some pain-related phenomenon.18 Modulation of these substrates will assist in the effectiveness of therapeutic interventions and will be discussed next. The SG contains an ascending gating mechanism to block nociceptive impulses from leaving the dorsal horn of the spinal cord. The first-order neurons for both nociceptive and nonnociceptive information synapse with second-order neurons in the SG. The second-order neurons for both types of information project to specialized neurons named T cells (transmission cells) in lamina V. For pain transmission to occur, T cells must be stimulated while the SG is inhibited. The input from A delta and C fibers stimulates the T cells and inhibits the SG (Figure 32-2). Therefore A delta and C fiber input opens the gate, allowing pain transmission to the higher centers. On the other hand, when the SG and T cells are both stimulated, the T cells are inhibited and the gate is closed to pain transmission. The input from nonnociceptive A beta fibers carrying information from pressoreceptors and mechanoreceptors stimulates both the T cells and the SG. Therefore A beta fiber input closes the gate, blocking pain transmission.17 There are at least two descending pain modulation systems. One involves the action of neurotransmitters, including serotonin, dopamine, norepinephrine, and substance P. High concentrations of brain serotonin108 and l-dopa (a precursor of dopamine)19 have been found to inhibit nociception, whereas norepinephrine appears to enhance nociception.20–23 The spinal mediators of descending nociceptive inhibitory influences include serotonin, norepinephrine, and acetylcholine (ACh). This may be relevant to the action of antidepressants in relieving pain in the absence of depression. Substance P is thought to be the neurotransmitter for neurons transmitting chronic pain.24 The second descending modulating system is mediated by neuromodulators—chemicals capable of directly affecting pain transmission. The neuromodulators include enkephalin and β-endorphin, which are referred to as endogenous opiates because they have morphine-like actions and are found in areas of the central nervous system (CNS) that correspond to opiate-binding sites. Endogenous opiates are believed to modulate pain by inhibiting the release of substance P. They have been shown to have a profound effect on nociception and mood.25–27 Their levels in the brain and spinal cord rise in response to emotional stress, causing an increase in the pain threshold and providing a possible reason that acute stress decreases acute pain.28,29 Although serotonin is not classified as an endogenous opiate, it exerts a profound effect on analgesia and enhances analgesic drug potency. High concentrations of serotonin lead to decreased pain by inhibiting transmission of nociceptive information within the dorsal horn,30,31 whereas low concentrations result in depression, sleep disturbances, and increased pain. The success of several therapeutic modalities, including noxious counterirritation (e.g., brief intense TENS or acupressure) and diversion (including hypnosis), is attributed to raising the level of endogenous opiates in the body.29 Acute pain is the normal predicted physiological response and serves as a warning. It alerts the individual that tissues are exposed to damaging or potentially damaging noxious stimuli. Acute pain is localized, occurs in proportion to the intensity of the stimuli, and lasts only as long as the stimuli or the tissue damage exists (1 to 6 months).32 Although acute pain is associated with anxiety and increased autonomic activity (increased muscle tone, heart rate, and blood pressure),33 it is usually relieved by interventions directed at correcting the injury. The pain experience is usually limited to the individual.34 Chronic pain is usually referred to as intractable pain if it persists for 6 or more months. It is defined as pain that continues after the stimulus has been removed or the tissue damage heals. Physiologically, chronic pain is believed to result from hypersensitization of the pain receptors and enlargement of the receptor field in response to the localized inflammation that follows tissue damage.35 Chronic pain is poorly localized, has an ill-defined time of onset, and is strongly associated with the subjective components outlined previously. It does not respond well to interventions directed solely at correcting the injury. Chronic pain patients frequently report other symptoms, such as depression, difficulty sleeping, poor mental and physical function, and fatigue. The effects of the pain experience extend beyond the individual and affect the family, the workplace, and the social sphere of the individual.34 Referred pain is felt at a point other than its origin. Pain can be referred from an internal organ, a joint, a trigger point, or a peripheral nerve to a remote musculoskeletal structure. Referred pain usually follows a specific pattern. For example, cardiac pain is frequently referred to the left arm or jaw; the referral pattern for trigger points is exact enough to be used as a diagnostic tool and is often used by physicians to diagnose pathology. Referred pain is the result of a convergence of the primary afferent neurons from deep structures and muscles to secondary neurons that also have a cutaneous receptive field.36,37 Although it is now recognized that all neuropathic pain results in abnormal activity within the CNS,38 pain initiated or caused by a primary lesion or dysfunction of the CNS39 is referred to as central neuropathic pain. The involvement of the nervous system can be at many levels: nerves, nerve roots, and central pain pathways in the spinal cord and brain. In this circumstance, there is permanent damage to the nervous system (usually a peripheral nerve) and likely anatomical reorganization of spinal terminations of surviving axons or ectopic activity from a neuroma that contributes paroxysmal, persistent input to the spinal cord.40 In addition to anatomical reorganization in the spinal cord, there could be some reorganization in the rostroventral medulla (RVM) as well, but more likely there is prolonged input to the RVM that sustains facilitatory influences that descend to the spinal cord. Less appreciated, descending facilitatory influences on spinal sensory processing could also be important to maintenance of chronic pain conditions, particularly those that persist in the absence of obvious tissue pathology.40 The onset of central neuropathic pain is usually delayed after the occurrence of the initial episode that results in damage to the CNS; onset of pain may occur during the phase of recovery from neurological deficits.41 Pain originating from a cerebrovascular incident and spinal cord injury usually begins weeks or months after the insult, whereas pain originating from tumors may take years to begin.38 Individuals with central neuropathic pain may have difficulty describing their pain and report burning, aching, pricking, squeezing, or cutting pain after cutaneous stimulation, movement, heat, cold, or vibration. A normally nonnoxious stimulus, such as moving clothing across skin, becomes agonizing. In some cases the pain begins spontaneously.42 Pain intensity varies, but it does seem to be associated to some degree with the location of the lesion.38 Allodynia (pain from normally nonnoxious stimuli) and dysesthesia are common, and one of the characteristic features of central neuropathic pain is that the clinical symptoms persist long after the stimulus has been removed. Central neuropathic pain is topographical. The site of the lesion determines the location of the symptoms. The pain may involve half the body, an entire extremity, or a small portion of one extremity.38 It is frequently migratory. Thalamic pain is the classic example of central neuropathic pain. Central neuropathic pain is difficult to treat. Surgery is not helpful for most individuals with central neuropathic pain, and medications have not been effective in permanently relieving the symptoms.11 Therefore the treatment of clients with central neuropathic pain stresses coping strategies and prevention of loss of activity and participation. The ideal management of a chronic pain patient is by a multidisciplinary approach, including disciplines such as internal medicine, neurology, anesthesia, nursing, psychology, pharmacy, rehabilitation medicine, physical therapy, occupational therapy, and others. The limitation of this approach is that access to such a wide range of specialists is often available only at large medical centers and special pain clinics, which restricts access to a limited number of patients. Allodynia is a product of the phenomenon of central sensitization.43 After injury, new axons sprout from the sympathetic efferent neurons. These fire spontaneously and, because they synapse on the cell bodies of the primary afferent neurons, cause them to fire as well. In addition, the dorsal horn neurons themselves become more excitable. They show an enlargement in their receptive field and become more sensitive to mechanical, thermal, and chemical stimulation. The result is an increase in the neuronal barrage into the CNS and the perception of pain with usually nonpainful stimuli.13 Complex regional pain syndrome (CRPS) is an example of pain that arises from abnormal activity within the ANS.44 CRPS has been classified into two distinct types39: CRPS type I (formerly reflex sympathetic dystrophy) follows mild trauma without nerve injury, and CRPS type II (formerly causalgia) follows trauma with nerve injury. CRPS type I generally begins within the month after the injury, whereas CRPS type II can occur any time after the injury.45 The main features of CRPS type I are constant burning pain that fluctuates in intensity and increases with movement, constant stimulation, or stress. There are also allodynia and hyperalgesia, edema, abnormal sweating, abnormal blood flow and trophic changes in the area of pain, and impaired motor function. CRPS type I is relieved by blocking the SNS, indicating that the pain is sympathetically maintained.45 CRPS type II occurs in the region of a limb innervated by an injured nerve. The nerves most commonly involved in CRPS type II are the median, sciatic, tibial, and ulnar; involvement of the radial nerve is rare. Pain is described as spontaneous, constant, and burning and is exacerbated by light touch, stress, temperature change, movement, visual and auditory stimuli, and emotional disturbances. Allodynia and hyperalgesia are common and may involve the distribution of more than one peripheral nerve. As with CRPS type I, edema, abnormal sweating, abnormal blood flow, trophic changes, and impaired motor function occur. The symptoms spread proximally and can involve other areas of the body. Evidence also points to sympathetic involvement in CRPS type II.45 The treatment of CRPS is complex and must be carefully coordinated among members of an interdisciplinary team including the neurologist (medications), psychologist (behavior), anesthesiologist (injections), and therapist (functional recovery). The therapist provides the core treatment to improve function. Therapists need to pay close attention to the following aspects of the disorder: (1) the degree of motor abnormalities, including restricted active range of motion (ROM), abnormal posturing, spasm, tremor, and dystonia; (2) true passive range restriction; (3) hyperesthesia and allodynia; (4) swelling and vasomotor changes; and (5) evidence of osteoporosis by radiograph.46 Please refer to Case Study 32-1 for interventions for clients with CRPS. Peripheral pain results from noxious irritation of the nociceptors. The character of peripheral pain depends on the location and intensity of the noxious stimulation, as well as which fibers carry the information into the dorsal gray matter. As noted previously, information carried on A delta fibers is sharp and well localized, begins rapidly, and lasts only as long as the stimulus is present, whereas information carried on C fibers is dull and diffuse, has a delayed onset, and lasts longer than the duration of the stimulus. The treatment of peripheral pain is covered in detail in Chapter 18. The management of central versus peripheral pain is determined by the type of pain—acute or chronic—and the clinical features present, including clinical localization; time of onset; laboratory study localization; response to analgesics, including narcotics; response to antidepressants; and response to nerve block or neurectomy.41 Differentiation among features will drive the treatment plan, but because some peripheral and central forms can coexist, diagnosis may be difficult. The multidimensional aspects of chronic pain make it important to evaluate the causes as well as the emotional and cognitive sequelae.47 Persistent pain is now considered to have a psychogenic component.48 The longer an individual has pain, the more a psychological component may become dominant. Many emotional factors can strongly influence pain, such as pain thresholds, past experiences with pain, coping styles, and social roles. The emotional experience that we perceive with pain reflects the interaction of higher brain centers and subcortical regions, such as the amygdala and cingulate gyrus (limbic system).49 Positron emission tomography of patients with chronic neuropathic pain demonstrates a shift of acute pain activity in the sensory cortex to regions such as the anterior cingulate gyrus.50 Understanding the physical limitations imposed by chronic pain is an area that therapists commonly assess; it is the mind-body connection that is often less articulated by the client and more difficult for the practitioner. Treatment of chronic pain should include a patient-centered approach, given the unique manifestations that occur in an individual’s response to pain. Patient-centered models, such as the ICF model, provide a framework that embraces a multidisciplinary team approach practiced in pain clinics. In such models, chronic pain has been noted to include psychological factors such as feelings of fear, anxiety, and depression,51 which are known to have the ability to modulate and exacerbate the physical pain experience.52 For example, a client with chronic pain who has the fear that movement will increase pain may alter his activity, causing muscular shortening, spasms, and a spiraling course of more pain and disability. The focus in treating clients with chronic pain should be on improving functional physical activity, decreasing peripheral nociception and central facilitation, and providing cognitive and behavioral strategies to help in resuming normal activities. Research has shown that pain memory does not provide an accurate measure of pain intensity.53 Therefore pain measurement tools are designed to provide information about the intensity, location, and character of a client’s symptoms at the time of the evaluation. This information can then be merged with the pain history, the disease or pathology history, and the physical findings to identify the cause of the pain. The disease or pathology management and its pain measurement will be the responsibility of the physician, whereas the movement limitations caused by the pain are the responsibility of the therapist. A number of pain measurement tools are available. These tools are used by professionals whose focus is pathology, as well as professionals whose responsibility is helping the patient to regain functional activities and life participation. The applications and limitations of several are discussed. Pain intensity rating tools are scales that have the client rate the current level of pain by marking a continuum or assigning a numerical value to the pain intensity (Figure 32-3). Each of the first three tools described here has been found to be reliable over time when used to measure pain that is present at the time of the rating. In general, however, clients who are depressed or anxious tend to report higher levels of pain and clients who are not depressed or anxious tend to report lower levels of pain on all three of these scales.54 With the visual analog scale (VAS), the client rates the pain on a continuum that begins with “no pain” and ends with “maximum pain tolerable.” This tool provides an infinite number of points between the extremes, making it sensitive to small changes in pain intensity. However, it has not been found reliable for individuals who have impaired abstract thinking skills55 and may be unable to translate their pain intensity into a corresponding point on a line. With a simple descriptive pain scale (SDPS), the client rates the pain on a continuum that is subdivided using descriptors that gradually increase in intensity. Sample descriptors are “no pain,” “mild pain,” “moderate pain,” “severe pain,” and “maximum pain tolerable.” This tool is more useful than the VAS for clients with impaired abstract thinking because it is easier for them to identify with the pain descriptors than with the line found in the VAS. However, clients have been found to favor the points corresponding to each descriptor rather than points between, resulting in a less sensitive tool than the VAS.56 With the Faces Pain Scale, the client selects one of seven schematic faces representing gradually increasing pain intensities. The scale begins with a face representing no pain and ends with a face representing the most pain possible. This tool is designed for use with young children who do not have the ability to use any of the three previous tools. The Faces Pain Scale has been found to be valid across cultural lines57 and to have a strong correlation with other pain measures.56 It is simple to use, does not require verbal skills, and requires little instruction. It has been used successfully with children as young as age 3 and with individuals who are limited in verbal expression. The client is asked to draw his or her symptoms on a schematic of the human body using a provided list of symbols (Figure 32-4). The result is a diagram describing the nature and location of the client’s pain, which can be compared with the client’s verbal report. In addition to providing a database, the pain drawing has been found to be useful in identifying individuals who have a heavy psychological or emotional component to their pain, making it helpful also in identifying clients who would benefit from further psychological evaluation.58 One of the most popular scales to rate pain quality is the McGill Pain Questionnaire (MPQ), which includes 20 categories of descriptive words covering the sensory (numbers 1 to 10), affective (numbers 11 to 15), and evaluative (number 16) properties of pain (Figure 32-5). Sensory properties are measured using temporal, thermal, spatial, and pressure descriptors. Affective properties are measured using fear, tension, and autonomic descriptors. Evaluative properties are measured using pain experience descriptors.59 Each word has a numerical value based on its position within its category. The client is instructed to “select the word in each category that best describes the pain you have now. If there is no word in the category that describes the pain, skip the category. If there is more than one word that describes the pain, select the word that best describes the pain.”59 The MPQ can provide the following types of information59: The MPQ has been studied extensively and found valid for adults with acute and chronic pain as well as for those with a variety of specific pathological states.60–62 It provides clues into the specific cause of pain because it describes the client’s symptoms. However, the MPQ does pose some disadvantages. It is time-consuming, requiring more time to complete than any of the previously described rating scales. Thus it is not appropriate for quick estimates of pain after treatment. Clients, especially children, are frequently unfamiliar with some of the descriptors and ask the evaluator to assist by defining words. However, reliability and validity of this test are based on examiner objectivity, and care must be taken to avoid the introduction of evaluator bias by helping the client to select appropriate descriptors.59 This issue can be dealt with by telling the client, “If you do not recognize a word, it probably does not apply to you.” Because a child’s description of pain is limited by a smaller vocabulary, Wilkie and associates63 have developed a verbal descriptor scale specifically for use with children (Table 32-1). Their list includes 56 words commonly used by children aged 8 to 17 to describe their pain experience. The word list is divided into the four categories found in the MPQ. The evaluators’ research has shown the list to be useful for children with a variety of diagnoses because it is relatively free of gender, ethnic, and developmental bias. TABLE 32-1 PEDIATRIC VERBAL DESCRIPTOR SCALE S, Sensory; A, affective; E, evaluative. Reprinted from Wilkie DJ, Holzemer WL, Tesler MD, et al: Measuring pain quality: validity and reliability of children’s and adolescents’ pain language. Pain 41:151–159, 1990, with permission from Elsevier Science.
Pain management
Defining pain
 A perceptual component: the client’s awareness of the location, quality, intensity, and duration of the pain stimulus
A perceptual component: the client’s awareness of the location, quality, intensity, and duration of the pain stimulus
 An affective component: the psychological factors surrounding the client’s pain experience, including the client’s personality and emotional state
An affective component: the psychological factors surrounding the client’s pain experience, including the client’s personality and emotional state
 A cognitive component: what the client knows and believes about the pain resulting from his or her cultural background and past pain experiences (both personal pain experiences and those of others)
A cognitive component: what the client knows and believes about the pain resulting from his or her cultural background and past pain experiences (both personal pain experiences and those of others)
 A behavioral component: how the client expresses the pain to others through communication and behavior
A behavioral component: how the client expresses the pain to others through communication and behavior
Pain anatomy
Pain transmission
Pain modulation
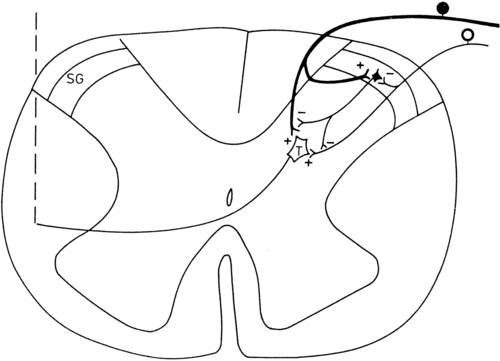
The gate control theory
Descending pain modulation system
Categorizing pain
Examination of the client with pain
Pain history
 Observation: Observation of the client from the moment of entry until (and sometimes beyond) the moment of exit from the clinic. By observing the client outside of the evaluation, the therapist is able to assess the client’s movement. The patient’s nervous system will accurately express itself to the therapist, especially when the patient is asked to focus attention on a topic other than pain and the patient is not aware that movement is being observed.
Observation: Observation of the client from the moment of entry until (and sometimes beyond) the moment of exit from the clinic. By observing the client outside of the evaluation, the therapist is able to assess the client’s movement. The patient’s nervous system will accurately express itself to the therapist, especially when the patient is asked to focus attention on a topic other than pain and the patient is not aware that movement is being observed.
 Origin and onset: Date and circumstances of the onset of pain. How did the pain start? Gradually or suddenly? Was there a precipitating injury? If so, what was the mechanism of injury? If not, can the client correlate the onset to a particular activity or posture?
Origin and onset: Date and circumstances of the onset of pain. How did the pain start? Gradually or suddenly? Was there a precipitating injury? If so, what was the mechanism of injury? If not, can the client correlate the onset to a particular activity or posture?
 Position: Location of the pain. Have the client demonstrate where the pain is located rather than relying on description alone. In addition to being more accurate, demonstration allows another observation of the client’s ability and willingness to move. Clients can also be asked to draw their symptoms on a schematic, such as the pain drawing, which is described later.
Position: Location of the pain. Have the client demonstrate where the pain is located rather than relying on description alone. In addition to being more accurate, demonstration allows another observation of the client’s ability and willingness to move. Clients can also be asked to draw their symptoms on a schematic, such as the pain drawing, which is described later.
 Pattern: Pattern of the pain. Is the pain constant or periodic? Does it travel or radiate? Which activities and postures increase or decrease the pain? Does medication or time of day have any effect on the pain? Have there been any recent changes in the pattern? Does the client believe that the pain is improving, worsening, or remaining the same?
Pattern: Pattern of the pain. Is the pain constant or periodic? Does it travel or radiate? Which activities and postures increase or decrease the pain? Does medication or time of day have any effect on the pain? Have there been any recent changes in the pattern? Does the client believe that the pain is improving, worsening, or remaining the same?
 Quality: Characteristics of the pain. Does the client use adjectives indicating mechanical (pressing, bursting, stabbing), chemical (burning), neural (numb, “pins and needles”), or vascular (throbbing) origin? Two tools for describing pain character are described later.
Quality: Characteristics of the pain. Does the client use adjectives indicating mechanical (pressing, bursting, stabbing), chemical (burning), neural (numb, “pins and needles”), or vascular (throbbing) origin? Two tools for describing pain character are described later.
 Quantity: Intensity of the pain. How has the pain intensity changed since the onset? Several methods that allow for monitoring change in pain intensity are presented later.
Quantity: Intensity of the pain. How has the pain intensity changed since the onset? Several methods that allow for monitoring change in pain intensity are presented later.
 Radiation: Characteristics of pain radiation. Does the pain radiate? What causes the pain to radiate? Can the radiation be reversed? How?
Radiation: Characteristics of pain radiation. Does the pain radiate? What causes the pain to radiate? Can the radiation be reversed? How?
 Signs and symptoms: Functional and psychological components of the pain. Has the pain resulted in any functional limitations? Has it caused any changes in the client’s ability to participate in life, including employment and recreational activities? Does the client’s personality contribute to the pain, or has the pain caused changes in the client’s emotional stability? Does the client benefit from the pain? How? It may be necessary to interview the client’s significant others or family members for an accurate picture.
Signs and symptoms: Functional and psychological components of the pain. Has the pain resulted in any functional limitations? Has it caused any changes in the client’s ability to participate in life, including employment and recreational activities? Does the client’s personality contribute to the pain, or has the pain caused changes in the client’s emotional stability? Does the client benefit from the pain? How? It may be necessary to interview the client’s significant others or family members for an accurate picture.
 Treatment: Previous and current medical and therapeutic treatment and its effectiveness, including medications, home remedies, and recommendations for movement activities. It is also important to determine the client’s attitude and expectations concerning therapy in addition to obtaining a treatment history.
Treatment: Previous and current medical and therapeutic treatment and its effectiveness, including medications, home remedies, and recommendations for movement activities. It is also important to determine the client’s attitude and expectations concerning therapy in addition to obtaining a treatment history.
 Visceral symptoms: Physical symptoms of visceral origin that can accompany and be responsible for the pain (Box 32-1). Visceral causes of pain require referral to the client’s physician for further investigation before the initiation of treatment by a therapist.
Visceral symptoms: Physical symptoms of visceral origin that can accompany and be responsible for the pain (Box 32-1). Visceral causes of pain require referral to the client’s physician for further investigation before the initiation of treatment by a therapist.
Pain measurement
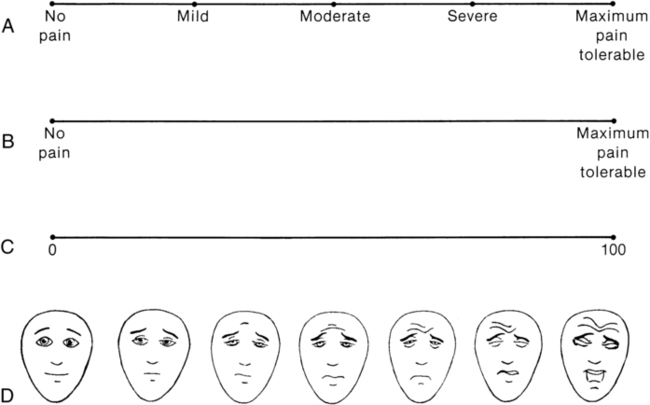
Measuring pain intensity
Visual analog scale.
Simple descriptive pain scale.
Faces pain scale.
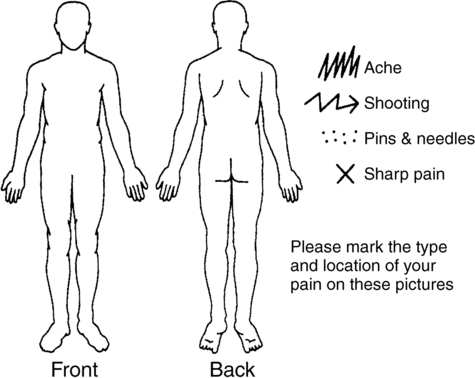
Localizing pain symptoms
Pain drawings.
Describing pain quality
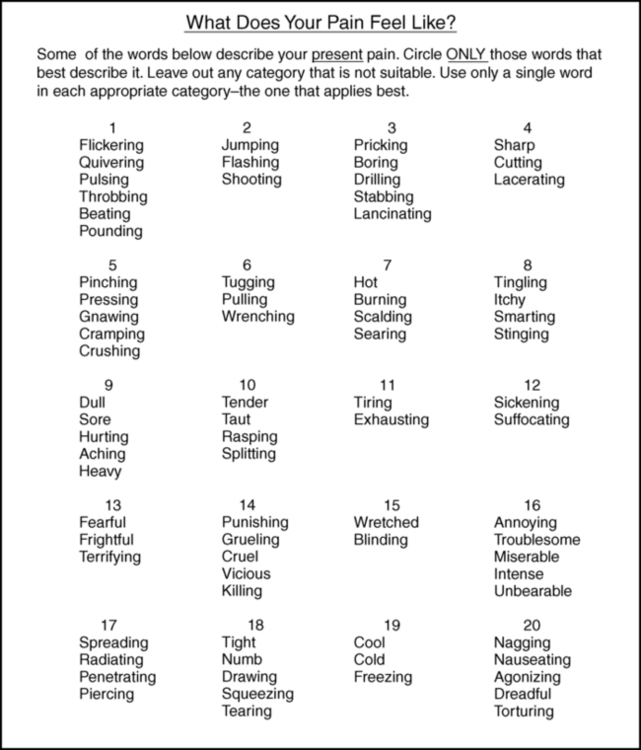
Mcgill pain questionnaire (mpq).
 A pain-rating index based on the sum of the values of all the words selected
A pain-rating index based on the sum of the values of all the words selected
 A pain-rating index based on the sum of the values of all the words in a given category
A pain-rating index based on the sum of the values of all the words in a given category
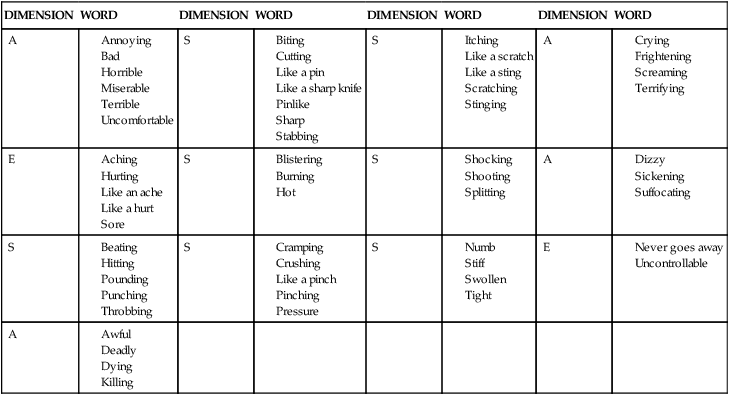
Pediatric verbal descriptor scale.

DIMENSION
WORD
DIMENSION
WORD
DIMENSION
WORD
DIMENSION
WORD
A
S
S
A
E
S
S
A
S
S
S
E
A
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pain management
Only gold members can continue reading. Log In or Register to continue