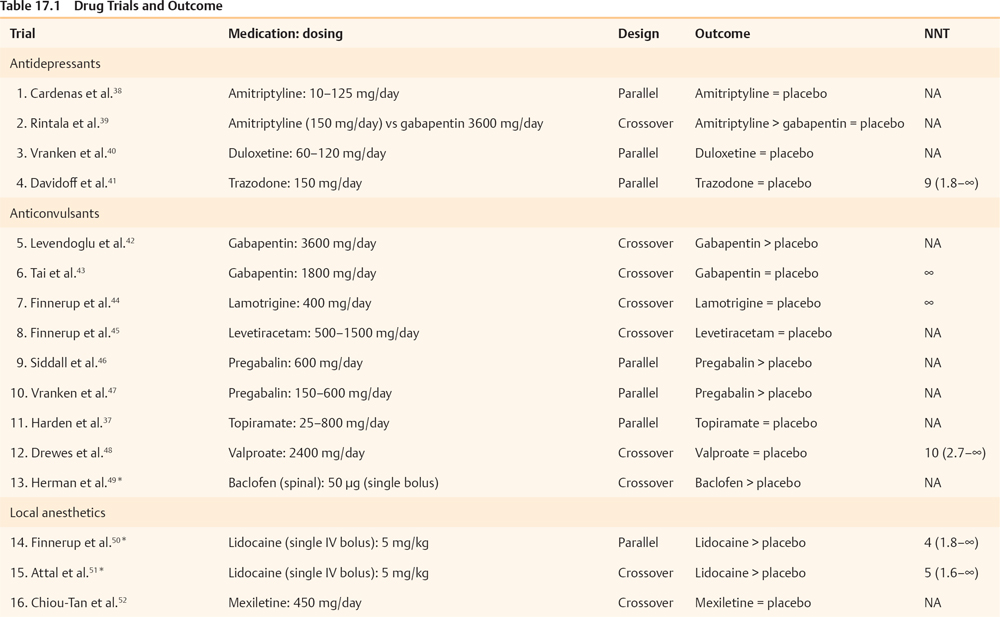
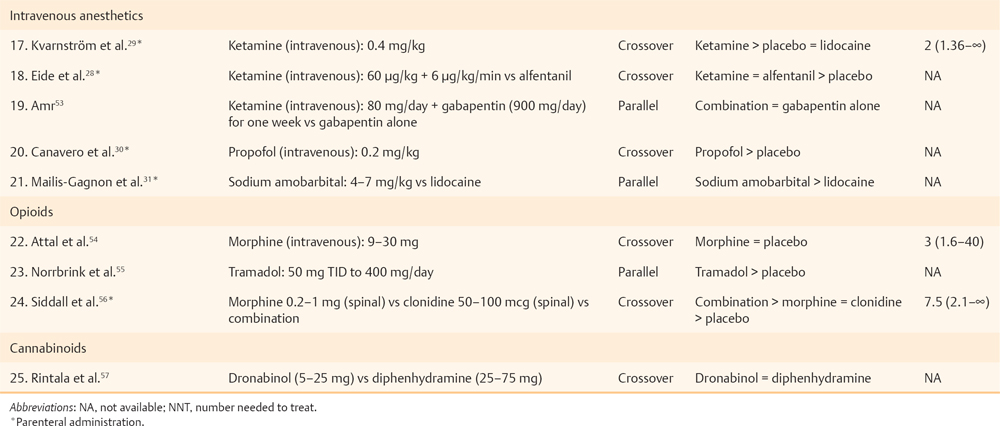
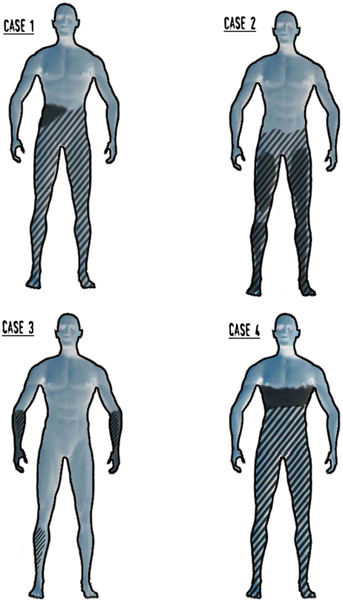
17 Key Points 1. Pain after traumatic or other forms of injury to the spinal cord is very common (estimated to affect 25 to 96% of patients). However, estimates of prevalence, severity, and duration of pain are highly variable in the published literature due to methodological and other differences between the studies. 2. SCI-associated pain can be nociceptive somatic (with musculoskeletal pain common in both the acute and the chronic stage of SCI), nociceptive visceral (originating from bladder, bowel, and kidney problems), neuropathic (above, at, and below level), or a combination. 3. SCI-related pain can be treated with medications, physical and occupational therapies, psychologically based treatments, and surgery. Generally, the treatment should be multimodal. Spinal cord lesions are usually traumatic, but spinal cord damage can also be the result of multiple other causes (iatrogenic, inflammatory, neoplastic, vascular or skeletal pathology, or congenital). Persisting pain is one of the commonest and most debilitating consequences of spinal cord injury (SCI).1 Following the inability to walk and bowel or bladder dysfunction, a significant number of individuals with SCI consider chronic pain as a very disabling complication.2 There is a strong association between pain and psychological factors or social disability. Indeed, psychological factors have a stronger association with pain than the medical condition per se in patients with SCI pain.3,4 In addition, the pain intensity is strongly correlated with concomitant sleep disorders,2 while pain, fatigue, and weakness are major contributors to social disability.5 Estimates of the prevalence, severity, and duration of pain after SCI are highly variable in the published literature. The variability is due to differences among the studies in regard to pain definitions, terminology, classification, inclusion criteria, and reporting methods, as well as etiological and demographic factors. An earlier evidence report,6 which reviewed 132 studies, found serious methodological limitations in most. Nevertheless, the report concluded that the prevalence of chronic pain after SCI varied from 40 to 75%, whereas pain was reported as moderate to severe in 25 to 60% of those with pain, was often associated with psychological and psychiatric comorbidity and was severe enough to impair daily function. A very recent review7 of the literature that used different inclusion criteria identified 42 studies, with reported prevalence ranging between 26 and 96%, unaffected by sex/gender, complete/incomplete SCI, or paraplegia/tetraplegia. A 5-year follow-up study8 reported prevalence of persistent severe pain up to 58%, which was, however, not associated with either the level or the type of injury. In a community survey of 384 patients with SCI, 79% of respondents reported current pain, which was significantly more common in persons with less education, unemployed, or not at school. Most common locations of current pain were the back (61%), hips and buttocks (61%), and legs and feet (58%). Upper extremity pain was experienced by 76% after the injury and by 69% of patients at the time of the survey. Those with tetraplegia were significantly more likely to have neck and shoulder pain than patients with paraplegia. On average, respondents reported a high level of pain intensity and a moderate level of pain interference with activities, and they rated treatments received for pain as being only somewhat helpful.9 A comprehensive taxonomy has been proposed by the International Association for the Study of Pain Task Force on pain after SCI,10 which has been helpful in subsequent studies. The taxonomy details mechanism of pain and the system as well as specific structure involved and classifies the pain as follows: nociceptive musculoskeletal or visceral and neuropathic above level, at level, and below level. Nociceptive musculoskeletal pain is common in both the acute and the chronic stage of SCI. Most of the time, upper extremity pain is attributed to overuse. Musculoskeletal spinal pain is due to fractures, surgical fixations, osteoporosis, or muscle spasms and tends to be more common after thoracic spinal injury and surgical procedures within 2 weeks after the lesion.11 Nociceptive visceral pain originating from bladder, bowel, and kidney problems is manifested by cramps and dull pain and is associated with nausea, autonomic reflex abnormalities, and dysautonomia. Autonomic dysreflexia is frequently associated with injuries above T6 and is manifested by episodic headaches, sudden increases in blood pressure, and cerebral hemorrhage.12 SCI neuropathic pain, like other neuropathic pain syndromes, can be idiosyncratic (i.e., not all patients will develop such pain13). Particularly the below-level pain (central pain) is caused by lesions of the primary somatosensory pathway passing through the ventrocaudal nucleus of the thalamus, especially the spinothalamic tract.14 The causative lesion may be massive or, to the contrary, minimal; sensory loss varies from minimal to complete anesthesia; pain onset can be immediate or delayed and can be ongoing, paroxysmal, or stimulus evoked; and different pain characteristics may have different underlying mechanisms. Above-level neuropathic pain is frequently related to compressive neuropathies (e.g., carpal tunnel syndrome), whereas below-level neuropathic pain is considered central pain secondary to the original injury. At-level neuropathic pain is related to nerve root or spinal cord compression or damage.15 Above- or at-level neuropathic pain is more frequently observed after cervical spinal injuries or central cord syndromes, and below-level neuropathic pain is more often associated with anterior cord lesions.16 Siddall and colleagues10,17 showed a prevalence of 41% at-level neuropathic pain, 34% below-level neuropathic pain, and 5% visceral pain in a 5-year follow-up study of patients with SCI. One of the main characteristics of at-level pain is its early onset, primarily within the first 3 months after the injury. The prevalence increases with high pain intensity and early onset. Two cardinal symptoms in at-level neuropathic pain are allodynia and pain of severe intensity. Examples of neuropathic pain are shown in Fig. 17.1. Fig. 17.1 Case 1: Complete anesthesia and paralysis below the T6 level. Pain is reported in the right flank (below-level). Case 2: Partial sensory and motor loss after T9-10 fracture with severe complaints of bilateral leg pain (below-level). Case 3: Incomplete SCI after C6-7 fracture/dislocation. Patient presents with generalized hypereflexia, minor weakness in the right arm and leg, and hypoesthesia in medial forearms and right leg, while pain involves both forearms (below-level). Case 4: T4 fracture with severe hyperalgesia, allodynia, and pain in the transitional zone (at-level) associated with complete anesthesia and paralysis below. Pain is shown in the dark-shaded areas whereas sensory loss is shown by the lined areas. Tasker et al. reviewed the essential features of 127 personal cases.18 Nearly two thirds of the injuries were traumatic in origin; three quarters of the patients were male; more than half of the patients were younger than 40 years of age; 42% of the lesions were cervical, followed by thoracolumbar lesions (37%). The authors pointed out that “it was curious that some patients with no detectable neurological deficit (4% of their series) had similar pain syndromes with some patients with complete cord transection.” In another series,19 onset of pain was reported immediately after the injury in 17% of the patients, less than a month in 13%, 1 to 6 months in 19%, 6 to 12 months in 8%, 1 to 5 years in 13%, and after more than 5 years in 2%. Pain in syringomyelia (idiopathic, associated with Chiari malformation or after traumatic SCI) deserves a brief mention. Spinal cord lesions of any type can lead to syringomyelia. Unusually long latencies between the injury and the development of pain should raise suspicion of cavitation. In one series,18 12.6% of patients with spinal cord lesions subsequently developed a syrinx characterized by delayed onset of pain a year or more than a year after the spinal cord lesion. A survey of the Canadian Syringomyelia Network participants during their 1996 convention reported a 97.5% prevalence of pain.20 The same study reported that pain was the sole symptom at onset in 59% of the patients and was cited as the primary cause of disability in 69% of the sufferers, and was rated as moderate in 70% of the patients, and severe in 11%. Several reviews have been published regarding the pathophysiological changes that occur after SCI.1,15,21–23 Early on, Levitt and Levitt24 studied central SCI pain in monkeys. Autotomy (as a manifestation of pain) appeared after section of the anterolateral quadrant of the cord or after hemisection with preservation of some sensation in ipsilateral nociceptive pathways, whereas pain never seemed to appear after posterior quadrant or funicular section. After further manipulations of the cord, the researchers concluded that the appearance of pain could not be related to any lesion of specific cord pathways. In humans, one of the earliest studies was conducted by Lenz et al.25 in a patient with SCI during deep brain stimulation. He reported missing receptive fields from the denervated area, increased numbers of thalamic neurons without a receptive field, expansion into the thalamic region of the deafferented part of neurons with receptive fields in the border zone of the deafferented area, mismatch between receptive and projected fields from stimulation, and spontaneous spikes from cells within the deafferented area. Pagni and Canavero26 reported a patient with a T9 spinal cyst whose single photon emission computed tomography (SPECT) showed diminished perfusion of the contralateral thalamus. Cyst resection resulted in elimination of pain and normalization of the SPECT, an effect that was also transiently produced by IV administration of propofol prior to surgery, suggesting that the responsible mechanisms are not necessarily always due to structural changes. In summary, in central pain after spinal cord or brain damage, the lesion could be anywhere in the neuraxis from the dorsal horn to the cerebral cortex, and most commonly is associated with interruption of the spinothalamocortical nociceptive pathways. In SCI, loss of balance between different sensory channels, loss of spinal inhibitory mechanisms, and/or pattern generators within the injured cord have been proposed as possible mechanisms. Canavero27 proposed that “irrespective of the location of the lesion, central pain is generated by disturbance in the normal oscillatory mechanisms between the cortex and the thalamus.” Increased burst activity may be related to loss of inhibitory drive on N-methyl-d-aspartate (NMDA) receptors or increased activity at NMDA receptor sites. The presence of glutamatergic hypertonus is suggested by the relief of central pain by ketamine,28,29 propofol,30 and barbiturates.31 Indeed, excitatory neurotransmitters across the dorsal horns play a special role in the development of at-level neuropathic pain.22 Such pain can subsequently progress to below-level neuropathic pain; therefore common mechanisms may be involved.32 Below-level neuropathic pain seems to be related to central mechanisms of pain,23 as discussed earlier. This type of pain is severe, can be evoked or spontaneous, and has a tendency to appear in later stages of the injury (> 2 years).15,17 This later onset indicates a slow neuronal degeneration process and subsequent hyperactivity secondary to deafferentation.17 Human studies have shown that below-level pain is quite frequent in partial lesions (more often in anterior lesions) and has been reported to occur in 50% of tetraplegics.17,33 We conducted a systematic literature review through PubMed using the MeSH terms “Spinal Cord Injuries” AND “Pain” limited to clinical trials. In Table 17.1, we present 25 clinical trials, ten of which included parenteral administration of drugs; therefore, their results are not clinically applicable. A qualitative assessment of the included studies shows that SCI barely responds to pharmacological approaches. Probably, some of the pharmacological strategies have failed to demonstrate a significant benefit due to small sample size (underpower studies). In our clinical perspective, we would recommend pregabalin for patients with concomitant anxiety, and antidepressants (we would opt for duloxetine) in patients with concomitant depression. Additionally, we would recommend, as a second line of treatment, the use of opioids under direct supervision by clinicians who are familiar with the aforementioned drugs. Ketamine could be used only for inpatients and always accompanied by concomitant treatment with a benzodiazepine to avoid potential hallucinatory effects. For curious clinicians, we recommend a simple but cleverly developed tool at PubMed called “Clinical Queries” (search by “Clinical Study Category”: spinal cord injury pain). The goal of physical therapies in general in SCI is to try to maintain, and if possible increase, strength, range of movement, balance, and coordination. Occupational therapies aim to increase functionality through performance of simple and complex real-life activities. However, few such therapies aim directly at pain of neuropathic origin. Desensitization (a procedure that brings hyperesthetic and allodynic skin into gradual contact with different textures, such as cotton, wool, etc.) aims to desensitize the skin, as in the areas of transitional zone pain and hyperpathia. In our experience, the effect is usually quite unsatisfactory. Oral medications seem to have a better but still limited chance of addressing skin hyperesthesia. Given anecdotal reports of the effect of acupuncture in some SCI pain, a study is currently in process as a multicenter randomized, controlled trial in Canada (clinical trials government identifier: NCT00523016) to test electroacupuncture versus sham acupuncture in SCI patients with burning pain. Chronic pain after SCI significantly interferes with activities of daily living, such as sleep, household chores, exercise, and work, and is reflected in negative coping, lower quality of life, and a significant incidence of depression. Rudy et al.34 found that psychological factors, such as self-efficacy perception, movement-related pain, fear, and cognitive coping, are strongly correlated with physical performance. Other factors, such as age, sex, and pain duration, were not associated with the magnitude of the physical performance. The main goals of treatment in general relate to improvement of the quality of life and early social reintegration and require some training in pain-coping skills and cognitive behavioral therapy, as well as adaptation to social, sexual, and communication skills. Considerations for surgical treatment should be given when the cord lesion creates disabling pain that fails to respond to conservative measures. The surgical options should be thoroughly discussed after consideration of efficacy, risk, and complexity of the procedure, as well as the nature of pain (because SCI pain has more than one component). The procedures are mentioned only briefly here because extensive review is beyond the scope of this chapter. The types of pain that require surgical consideration13 are ongoing (steady) burning, dysesthetic pain and intermittent paroxysmal shooting pains (neuralgic pains), below the level of the injury (central pains). A summary13 of invasive procedures follows: rhizotomy, preferably percutaneous, may help with “single root pain,” particularly to relieve allodynia in a single root distribution. Cordotomy may work better in radicular or paroxysmal pain (but not steady ongoing pain), but the effect may decrease with time. In one series, pain returned in 6/25 cases13 anytime between 1 and 21 years after the procedure. Cordectomy relates to two procedures: removing a segment of the spinal cord, or cord transection above the level of the SCI. Such procedures seem to work better for lesions below the T10 level and may affect both steady, ongoing pain and paroxysmal pains. Dorsal root entry zone (DREZ) lesion is a rather popular procedure for at-level pain, though it has also been reported in some patients to ameliorate below-level ongoing dysesthetic pains.
Pain after Spinal Cord Injury
 Epidemiology
Epidemiology
 Clinical Manifestations
Clinical Manifestations
 Pain Mechanisms Underlying Spinal Cord Injury Pain
Pain Mechanisms Underlying Spinal Cord Injury Pain
 Therapeutic Approaches to Spinal Cord Injury Pain
Therapeutic Approaches to Spinal Cord Injury Pain
Pharmacological Management
Physical and Occupational Therapies
Psychological Approaches
Invasive Treatments
Pain after Spinal Cord Injury