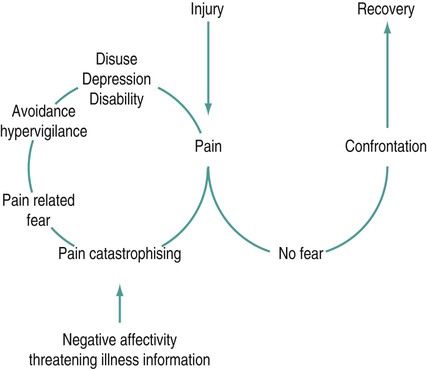
Lester Jones, G. Lorimer Moseley and Catherine Carus (Case study development) There are several key parts of this definition. • Firstly, although the nociceptive system is critical for detecting dangerous stimuli and alerting the brain, pain is not simply the transmission of noxious sensory information (nociception). Rather, pain has potentially profound cognitive and emotional influences, just like other perceptual states (even vision – think of your favourite visual illusion and notice that what you see is not necessarily an accurate reflection of the light that is hitting the light receptors on your retina). • Secondly, pain is not a measure of tissue damage, which means that tissue can be injured but not painful, and painful but not injured. • Thirdly, pain is about threat to body tissue, not about a particular mode of sensory input. This sets pain apart from other information that is sent from our tissues to our brain: the so-called somatic senses. It is this threat-specific quality that makes pain critical for protection and preservation of the body: pain seems to be the conscious component of a complex defence system. • Finally, there are many unconscious components of this defence system, all of which can influence each other and influence pain. Thus, pain is a fundamentally conscious process, which is preceded and accompanied by a range of responses, most of which are not conscious. According to this model of pain, when someone is in pain, we can be sure that their brain is concluding that tissue is in danger and that they should take some sort of action to get the tissues out of danger (Wall 1999; Butler and Moseley 2003). This section will discuss the physiology of pain under three categories: The peripheral nervous system is well studied but not completely understood. Many types of neurones in the periphery are thought to contribute to nociception (Meyer et al. 2006). The most important of these neurones are probably Aδ and C fibres – conventionally called nociceptors – although many Aδ and C fibres also respond to non-noxious inputs (Craig 2002). For the sake of clarity, we will classify Aδ and C fibres as primary nociceptors (see Table 17.1). Table 17.1 (Reproduced from van Griensven (2005); see also Craig (2002) and Meyer et al. (2006).) Many brain areas are involved in pain. Most often, the thalamus, insula, primary (S1) and secondary (S2) somatosensory and prefrontal cortices are involved. These areas are called the pain matrix (Apkarian et al. 2005) (Figure 17.1). However, these areas are never the only areas involved and they are not always all involved – the pattern of brain activation varies greatly between, and within, individuals. In an attempt to clarify the potential roles of different brain areas in pain, some authors conceptualise two systems. The medial nociceptive system includes the medial thalamic nuclei, anterior cingulate and dorsolateral prefrontal cortices. It is described as slow and only broadly somatotopic, which means it does not have the capacity to code in detail the location in the body at which the stimulus occurred. Activity in this system has been proposed to subserve the affective-emotional dimensions of pain, i.e. the unpleasantness of pain rather than the intensity and quality. This aspect of pain may have value to the person experiencing pain by triggering behaviour that demands other people’s attention (Goubert et al. 2009). The lateral nociceptive system includes the lateral thalamic nuclei and the primary (S1) and secondary (S2) somatosensory cortices. It is described as fast and is highly somatotopic, which means it is able to code, in great detail, the tissue location at which the stimulus occurred. Activity in this system is proposed to subserve the sensory-discriminative aspects of pain, i.e. the intensity of the pain and the sensory characteristics of the stimulus (e.g. warm, sharp, deep, superficial). The intensity and unpleasantness of pain can be independently manipulated (e.g. Moseley 2007a). Melzack (1990) proposed the neuromatrix theory as a way of conceptualising how myriad inputs and factors affect pain and nociception (Figure 17.2). Inputs and factors in that theory include previous learning and past experiences, immune and endocrine states and responses, activity in the autonomic nervous system, and information coming from nociceptors and other sensory receptors. The theory suggests that pain will occur when all these elements are evaluated and a specific network of neurones in the brain is activated. Melzack calls each particular network of neurones a neurosignature (or ‘neurotag’ (described by Butler and Moseley (2003)), which is analogous to the representation concept used in cognitive neuroscience literature (see Damasio (2000) for a review of representation theory). Remember that this is a theory and that the brain physiology underpinning pain and consciousness is not well understood. Peripheral mechanisms that increase the pain evoked by a standardised stimulus include the presence of inflammatory mediators (including proinflammatory cytokines, bradykinin, prostaglandins), decreased circulation (which increases the local concentration of H+ ions), the presence of immune mediators and activation of certain genes (Figure 17.2) (see Meyer et al. (2006) for an exhaustive review of peripheral mechanisms of modulation). Inflammatory mediators are released by tissue damage and by activation of nociceptors (neurogenic inflammation). This sensitisation, driven by peripheral mechanisms, is called peripheral sensitisation and the hyperalgesia that follows is called primary hyperalgesia. There are other mechanisms in the dorsal horn that can sensitise the nociceptive system. Wide-dynamic-range neurones in the laminae of the dorsal horn provide an interaction of the processing for nociception and other sensory information. This may be important in how people describe their pain, sometimes referred to as the ‘quality’ of pain. It also provides a potential mechanism for further sensitivity of the nociceptive system, whereby the responses to normally non-painful stimuli, such as touch, are now painful. This may be because the wide-dynamic-range neurones synapse with sensitised neurones. Another mechanism of sensitisation involves the sprouting of neurones across laminae. Such sprouting may link a non-nociceptive peripheral nerve fibre (e.g. Aβ fibre) with an interneurone that would normally respond to noxious stimulation. This is probably more likely to occur after peripheral nerve injury or death. Butler and Moseley’s (2003) Explain Pain discusses these mechanisms in an accessible, but reasonably detailed, way. Supraspinal mechanisms that enhance the sensitivity of the CNS include influences, mediated by the rostroventral medulla, on the inhibitory transmission affecting the spinal cord. Current research is looking not only at descending modulation, but also on intracortical modulatory processes. Imaging studies have associated pain processing with activity in multiple brain areas, commonly including the thalamus, anterior and posterior cingulate cortex, other areas of the limbic system and the medial prefrontal cortex (Bushnell and Apkarian 2006; Schweinhardt et al. 2008; Wiech et al. 2008). The attributed function of these brain areas supports a role in the cognitive modulation of pain (see below). The pain associated with nerve damage is known as neuropathic pain and may include descriptions such as ‘burning’ or ‘electric shock’. CNS damage, such as stroke, can result in centrally-mediated neuropathic pain that reduces the inhibition of the nociceptive system. Peripherally, damage to an axon or the myelin covering of a nerve, can lead to spontaneous transmission of impulses from the damaged area or the dorsal root ganglion (DRG). This may also be driven, in part, by loss of inhibition, specifically through alterations of chloride homeostasis affecting gamma amino-butyric acid (GABA)-mediated inhibition (see review of mechanisms in De Koninck (2009) and also Sandkühler (2009)). Such damage will usually result in hypersensitivity to mechanical input. This sensitivity forms the basis for tests such as Tinel’s sign – sometimes considered as an indicator of neuropathic pain. There is mounting evidence that interactions between the immune system, the endocrine system and the autonomic nervous system are important in all types of pain, including neuropathic pain (Watkins and Maier 2000). Anecdotal evidence that somatic, psychological and social factors modulate pain is substantial – sport- and war-related stories are common (see Butler and Moseley (2003) for several examples). However, numerous experimental findings corroborate the anecdotal evidence (see Fields et al. (2006) for a review of CNS mechanisms of modulation and Poleshuck and Green (2008) for a review of the impact of socioeconomic disadvantage and pain). Despite the wealth of data, consensus is lacking: some data suggest that attending to pain amplifies it and attending away from pain nullifies it; others suggest the opposite. This may be explained by the existence of different modes of selective attention (see Legrain et al. (2009) for review). Most likely, the effect depends on the coping style of the individual and the wider context of the experiment or situation. Anxiety also seems to have variable effects on pain. Some reports link increased anxiety to increased pain during clinical procedures (Schupp et al. 2005; Klages et al. 2006) and during experimentally-induced pain (Tang and Gibson 2005), but other reports suggest no effect (Arntz et al. 1990; Arntz et al. 1994). Relevant reviews conclude that the influence of anxiety on pain is probably largely dependent on attention (Arntz et al. 1994; Ploghaus et al. 2003). Expectation also seems to have variable effects on pain. As a general rule, expectation of a noxious stimulus increases pain if the cue signals a more intense or more damaging stimulus (Fields 2000; Sawamoto et al. 2000; Keltner et al. 2006; Moseley 2007a; Moseley and Arntz, 2007) and decreases pain if the cue signals a less intense or less damaging stimulus (Pollo et al. 2001; Benedetti et al. 2003). There are several informative reviews on the influence of expectation on pain (Fields 2000; Wager 2005). The common denominator of the effect of attention, anxiety and expectation on pain seems to be the underlying evaluative context, or meaning, of the pain. That is demonstrated by the consistent effect that some cognitive states seem to have on pain. For example, catastrophic interpretations of pain are associated with higher pain ratings in both clinical and experimental studies (see Haythornwaite (2009)). Believing pain to be an accurate indicator of the state of the tissues is associated with higher pain ratings (Moseley et al. 2004), whereas believing that the nervous system amplifies noxious input in chronic pain states increases pain threshold during straight leg raise (Moseley 2004). The social context of a noxious stimulus also affects the pain it evokes. For example, when men have blood taken by a woman, it hurts less than when it is taken by another man (Levine and De Simone 1991). The effects are variable but, again, seem to be underpinned by the underlying evaluative context or meaning (see Butler and Moseley (2003) for a review of pain-related data and Moerman (2002) for exhaustive coverage of the role of meaning in medicine and health-related interactions). This section uses the WHO ICF as a framework for assessment and treatment planning of patients in pain. This framework is advocated by the IASP (Wittink and Carr 2008). The WHO ICF focusses on how a person is functioning and evaluates outcomes using the person’s actual performance in the real-life environment. This makes it especially useful for those patients where pain relief is not the only, and possibly not the most important, objective. The WHO ICF has three components: the body (function and structure); activities and participation; and contextual factors (e.g. environmental factors that might influence function). Each component is classified at a level of severity (none, mild, moderate, severe, complete) and interactions between components are evaluated (WHO 2002). As it relates to people with pain, the three components of the ICF broadly mirror the components of the biopsychosocial model, which considers tissue-based, psychological and social influences on pain. Most therapeutic processes will start with an interview. The interview aims to capture information about the person in terms of the ICF. Physiotherapy interviews have conventionally focussed on symptoms: location, intensity, quality and temporal patterns, and signs. This information is very important, but it is not sufficient. According to the ICF this information relates to the body, the biopsychosocial model’s equivalent of the tissues. However, we advocate, as do other reviews in this area (e.g. Gifford et al. 2006) that the interview must also provide information about activities and participation and contextual factors (i.e. the ‘psychosocial’), and how these issues may interact with pain and the state of the tissues (Goldingay 2006a, 2006b). The aim of the interview then is to identify factors from across biopsychosocial domains which activate or sensitise the nociceptive or pain system (see Table 17.2). Table 17.2 Structured interview for biopsychosocial assessment Reproduced from Goldingay (2006b), with kind permission of S. Goldingay and the CNS Press. Visual analogue scales (VAS) and numerical rating scales (NRS) are most common and useful. A visual analogue scale consists of a horizontal line, usually 100 mm long. At each end is a term of reference for the patient, called an anchor. The left anchor is usually ‘No pain’ and the right ‘worst pain’ or ‘worst pain imaginable’. The patient marks a point on the line in answer to a question about their pain and the distance from the mark to the left anchor is used as a measure of their pain. A NRS uses numbers instead of a line, such that 0 = ‘no pain’ and 10 = ‘worst pain’. The VAS is probably more sensitive to change, less vulnerable to perseveration (remembering what you said last time and responding the same way) and more difficult to measure. The NRS is easier to use clinically and is probably sufficiently sensitive to detect clinically meaningful changes [a clinically meaningful change is usually considered to be about two points on a ten-point scale (McQuay et al. (1997))]. Several tools assess the quality of pain. A simple tool is a VAS, with anchors reflecting the unpleasantness of pain. The most widely used is the short form of the McGill Pain Questionnaire (MPQ) (Melzack 1975a). The MPQ lists a variety of words that are grouped as being about the sensory-discriminative aspect of pain (e.g. sharp, burning, intense), the affective aspect of pain (e.g. punishing) or its evaluative context (e.g. annoying). There are many other measures that emphasise different aspects of pain. Although self-reporting measures are behavioural measures, they are generally considered as a separate category. Non-self-reporting behavioural measures are useful as a corroborator of self-reporting measures, when the patient is unable to use the measure (e.g. children) or when the clinician considers the patient’s report to be dubious. Behavioural measures include observation (by a covert or overt observer) and performance (e.g. functional, endurance, speed or load tests). Full review of behavioural measures is beyond the scope of this chapter, but the interested reader is referred to the Handbook of Pain Assessment (Turk and Melzack 2001) and Manage your Pain (Nicholas et al. 2007) for examples. The importance of evaluation of the threat to body tissue has been mentioned earlier. Inherent to this evaluation is fear of movement and (re)injury. A large amount of research has investigated the role of pain-related fear avoidance behaviour. There are many specific findings that relate fear and catastrophic thought processes to pain intensity, disability and self-efficacy but the principle can be summarised thus: people who have heightened concerns about pain, its cause and its consequences, often have a sense of helplessness and adopt a passive coping style. As a result, they have a lower criterion for a movement or activity to be considered potentially painful or potentially injurious. They therefore avoid those movements and activities – ‘fear-avoidance’. Avoidance of activity leads to disuse and hampers healing and recovery, leaving the person in a vicious cycle of progressive depression, disuse and disability (Figure 17.3). According to the fear-avoidance model, most people do not follow this path because they have a more accurate and appropriate level of concern and thus do not tend to avoid movement and activity (Vlaeyen and Linton, 2000). See Vlaeyen and Linton (2000) for a review of fear-avoidance beliefs and pain and Sullivan et al. (2001) for a review of catastrophic thought processes and pain.
Pain
Introduction
The physiology of pain
Activation of the nociceptive system
Peripheral transmission
Characteristic
Aδ
C
1. Conduction speed*
Fast (4–36m/s)
Slow (0.4–2m/s)
2a. Accommodation (adaptation)
Fast
Slow
2b. Pain duration
Brief
Long
3a. Receptive field
Small
Large
3b. Localisation
Precise
Diffuse
4. Sensory quality
Sharp, pricking
Aching, dull, burning
5. CNS response
Reflex, analysis
Emotional, suffering
Brain transmission
Parallel processing
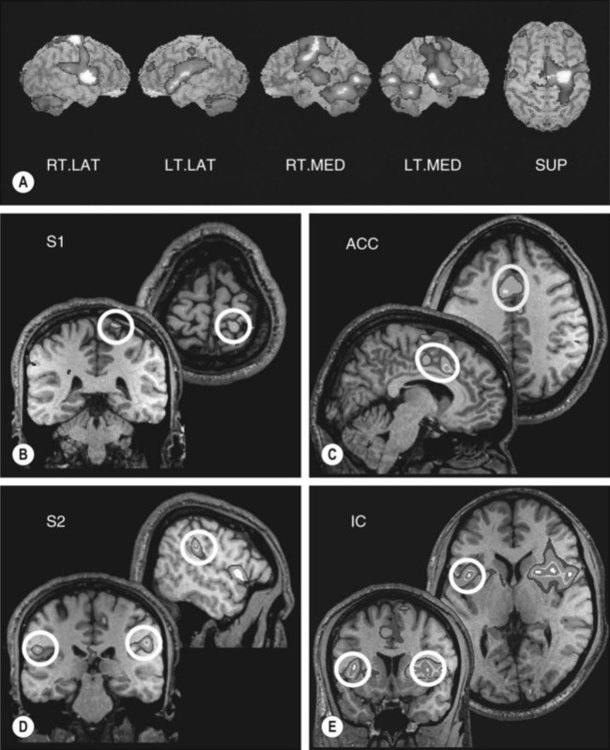
Different aspects of pain seem to involve different brain areas
Multiple inputs: The neuromatrix theory
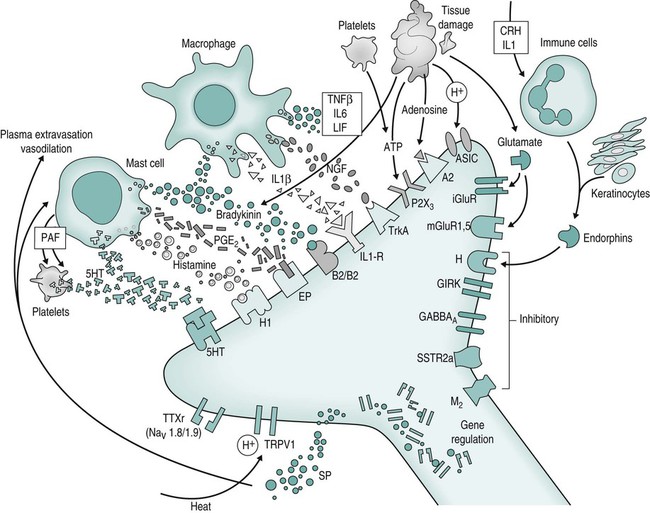
Sensitisation of the nociception/pain system
Peripheral mechanisms
Spinal cord mechanisms
Dorsal horn mechanism
Brain mechanisms
Neuropathic pain
Pain modulation via psychological and social influences
Anxiety
Expectation
Social context
Assessment and measurement of pain
The WHO international classification of functioning, disability and health (WHO ICF)
The interview
Area of examination
Information gained
Orientation
Nature and location of symptoms; patient’s story from onset to present, expectations of physiotherapy, questions and concerns about problem
Previous intervention
Investigations and understanding, treatment effects, advice received, causal beliefs
Medical history
Comorbidity and effect on function, special questions (red flag screening), medication
Effects on function and participation
Current social and employment situation, typical day, effects on work, restrictions on activity, assistance required, aids and adaptations, downtime, sleep
Coping strategies
Current coping strategies: active and passive, perceived consequences of change (e.g. increasing activity, exercise, pacing)
Socio-economic
Effect on finances, benefits, medico-legal
Effect on family
Beliefs, responses, nature of support provided
Emotion
Nature and extent, effect on motivation
Pre-examination
Body chart, behaviour of symptoms
Measures and scales
Self-reporting measures
Behavioural measures
Measuring potential impact of beliefs and thoughts
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pain








