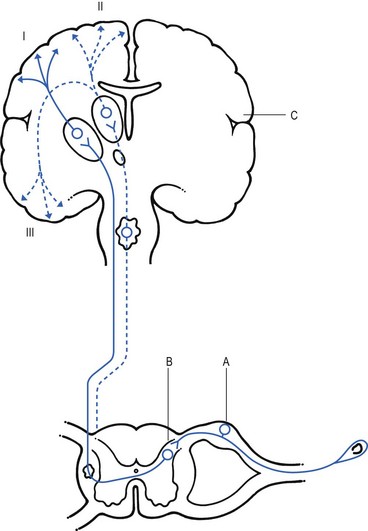
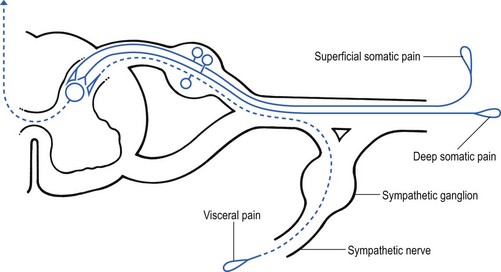
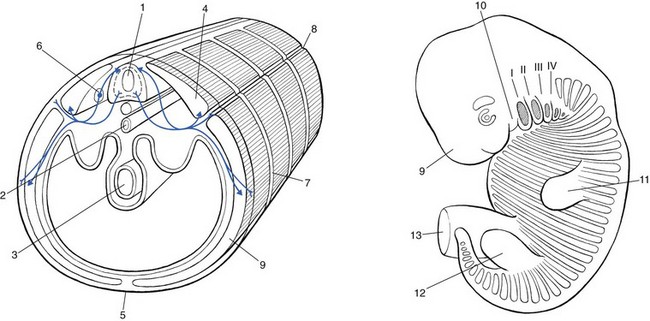
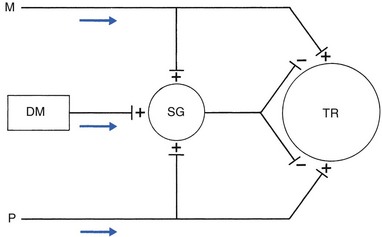
1 Pain is the presenting symptom in almost every orthopaedic patient. A complaint of pain is always indicative of some variety or degree of dysfunction1 and results from a combination of physical and psychological causes, although sometimes one or the other predominates. All pain must be regarded as real. Pain entirely devoid of somatic cause is labelled ‘psychogenic pain’: although no peripheral tissue damage exists, the pain is just as distressing as somatic pain2 (see Section 16). The taxonomy committee of the International Association for the Study of Pain defined pain as: ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’.3 Pain is thus not a ‘primary sensation’ in the sense that smell, taste, touch, vision and hearing are, but is an ‘emotional state’, like sorrow, love or hate. The consequence is that it is extremely difficult to explain one’s pain to another person. This is reflected in the numerous words that patients use to describe intensity and quality of pain: twinge, ache, distress, discomfort, soreness, cramp, suffering, misery, agony, torment, anguish.4 The fact that pain is always a subjective experience provides the first difficulty in its use in diagnosis. The language used is not always easy to understand, and the examiner usually needs a high level of competence and understanding to translate patients’ subjective descriptions into more objective and useful statements. The intensity of pain does not depend only on the intensity of irritation of the peripheral nociceptive system (receptors and their afferents). Centripetal transmission of peripheral nociceptive stimulation is subject to varying degrees of facilitatory and inhibitory modulation at different synapses during its course to the cerebral cortex. An important modulation site, of major concern to the orthopaedic physician, is the gateway synapse in the basal spinal nucleus, but there are also modulation systems in the spinal grey matter, in the thalamus and in the cerebral cortex itself.5 Nociceptive receptors are defined as nerve endings that are sensitive to noxious or potentially noxious (mechanical and chemical) stimuli. The perceptual aspect of the nociceptive system consists of unmyelinated free nerve endings, distributed three dimensionally throughout skin, subcutaneous and adipose tissue, fasciae, aponeuroses, ligaments, tendons, muscles, periosteum and bone.6,7 Clinically, three distinct areas of pain perception may be considered: the skin (superficial somatic pain); the locomotor system (deep somatic pain); and the viscera (visceral pain). Of these, only the skin is adapted to localize pain exactly in the region of injury. Deep somatic and visceral pain are often felt in unusual locations (see Referred pain, p. 6). In normal circumstances, this nociceptive receptor system remains largely inactive. The unmyelinated free nerve endings are depolarized only by the application of mechanical forces sufficient to deform or damage the tissue that contains them or after exposure to sufficient concentrations of irritating chemical substances (lactic acid, serotonin, prostaglandins and histamine), released from local inflammatory cells and from the peripheral terminals of the primary afferent fibres themselves.8–10 Another important influence on nociceptor sensitivity is the pH of the tissue. High local concentrations of protons are known to occur in inflammation and the consequent reduction in pH contributes to the sensitization of nociceptors.11,12 Nerve impulses generated at the nociceptive receptor system are delivered into the spinal cord by small myelinated and unmyelinated nerve fibres (5 µm or less in diameter), that mainly belong to the Ad and C groups of afferent nerve fibres (Fig. 1.1). Their cell bodies are located in the dorsal root ganglia of the spinal nerves. The very small diameter of the C nerves explains their slow conduction velocity (1 m/s), and their extreme sensitivity to blockade by local anaesthetic drugs. The myelinated Ad fibres are slightly larger and have a faster conduction velocity (10 m/s).13 The nociceptive afferents enter the spinal cord, where they divide into short ascending and descending branches, before they terminate at synapses on various groups of relay neurones in the dorsal horn of the spinal grey matter. Most of the connections are to the neurones in the basal spinal nucleus (at the base of the dorsal horn).14,15 The efferents of these cross the cord obliquely to turn upwards on the contralateral side and form the anterolateral spinal tract, which connects the basal spinal nucleus with the thalamic nuclei and has therefore traditionally been called the ‘spinothalamic tract’. Most of the fibres in this tract, however, do not directly ascend to the thalamus without interruption, but instead synapse with neurones in the brainstem reticular system, while others re-enter the spinal grey matter to synapse with internuncial neurones.16 However, the majority of the ascending nociceptive inputs terminate (sometimes after crossing several synapses) in the thalamic nuclear relay sites.17 It should be emphasized that not only do the neurones in the thalamic centres respond to peripheral noxious stimulation but they can also be activated by mechanoreceptor peripheral stimulation (see Pain modulation systems below). The axons of the thalamic nuclei then ascend to the neurones of the cerebral cortex. Three thalamocortical projections can be defined: those responsible for perception; those related to the emotional experience; and those responsible for memory.18 The first project to the superior paracentral region of the cerebral cortex and seem to contribute to the so-called ‘perceptual component’ of pain – the patient’s ability to perceive whereabouts (in which segment of his body) the pain is localized.19 The activation of the second thalamocortical projection system, projections that pass from the medial and anterior thalamic nuclei to the frontal lobes, evokes the emotional disturbances related with pain.20 Thus, a stimulus ‘hurts’ only when the nociceptive afferent projections arrive at the frontal cortex. A third thalamocortical projection system links some of the medial thalamic nuclei to the cortex of the ipsilateral temporal lobe. Here the recent and long-term memory storage systems of the brain are located.21,22 A fourth projection system exists which relates some thalamic nuclei to subjacent hypothalamic nuclei in the ventral diencephalon. It is very probable that this thalamohypothalamic system provides the means whereby nociceptive afferent activity entering the brain evokes the complex of visceral reflex (cardiovascular and gastrointestinal) effects and hormonal changes that are so often associated with the experience of pain.23 There are both peripheral and central pain modulation systems. One of the most important sites at which a synaptic modulation operates from both the peripheral and central sources is at the synapses in the basal spinal nucleus. In 1965, Melzack and Wall,24 basing their theory mainly on the work of Noordenbos,25 published an article entitled: ‘Pain mechanism: a new theory’. They called their concept of peripheral pain modulation the gate control theory (Fig. 1.2), which is based on three premises: Fig 1.2 Gate control theory (after Melzack and Wall24): P, small nociceptive fibres (pain); M, large mechanoreceptive fibres; TR, transmission cells (relay neurones in the basal spinal nucleus); DM, descending modulation; SG, substantia gelatinosa; +, excitatory effect; –, inhibitory effect. • Afferent nerves contain two types of fibres: small fibres (P), as described above, and large-diameter afferents (M), which are derived from the various mechanoreceptors in the articular capsule, ligaments and muscle spindles. These fibres produce information about static joint position, pressure changes in the joints, joint movement and stresses that develop in the joint at the extremes of movement. The fibres of mechanoreceptor transmission have a lower stimulation threshold and a faster conduction velocity than the smaller and mostly unmyelinated fibres of the nociceptive system. • In the substantia gelatinosa (SG) of the dorsal horn, both afferent systems converge and interrelate, with the overall effect that the large-diameter afferents have an inhibitory effect on the relay neurones located in the basal spinal nucleus. This inhibition is presynaptic and is reduced only when there is a massive input from the small nociceptive afferent fibres. The latter thus facilitates central transmission of pain. The interaction between both systems is gate control: impulses travelling along the larger fibres close the gate, and those in the small fibres open the gate so that impulses to thalamus and cortex can pass through. • The activity of the gate is not only modulated by impulses from nociceptive and mechanoreceptor systems but also receives a descending and regulating feedback from the reticular system, the thalamus and the cerebral cortex. Awareness of pain is also modulated at the central projection systems. A modulation system at the reticular formation in the brainstem exerts a continuous inhibitory effect on the projection neurones in the spinal nucleus ganglion via the reticulospinal tract, which is discharging continuously at varying frequencies throughout life.26 The inhibitory effect on nociceptive afferent transmission is augmented when the attention of the patient is distracted from the site of pain. This is what occurs when another painful site elsewhere in the body is stimulated (counter-irritation), when the patient concentrates on work or other activities or when hypnosis is induced.27 The inhibitory effect of this reticular system also increases when the blood concentration of catecholamines is very high, as can be the case in states of great emotional tension.28 Also some drugs (chlorpromazine, diazepam and morphine) may selectively increase the activity of the reticular neurones that operate this inhibitory system.29 Inhibitory reticular activity is depressed and pain is enhanced when attention is concentrated on the painful site, or following the administration of barbiturates, caffeine or theophylline.30 In 1905, Sir Henry Head described referred pain in the abdominal wall caused by a visceral disease.31 Using the dermatological appearances in herpes zoster, he constructed schemes of segmental innervation of the skin.32 He also described dermatomic zones that became painful in the event of provocation of a related visceral structure. His theory of pain reference was built on the concept of the segmental organization of the human body and its nociceptive system. Further experiments in this sphere were conducted by Sir Thomas Lewis in 1936.33 In 1938 and 1939, Kellgren published the results of a systematic examination of the phenomena of referred pain, demonstrating segmental radiation and failure to cross the midline.34,35 His experiments were confirmed by others.36–38 Later, the concept of segmental reference of pain was refined39,40 and exact borders of the different dermatomes mapped out.41–44 The fact that referred pain is an error in perception was first pointed out by John Hunter in 1835 (cited by Cyriax).45 It was obvious that if pain is felt elsewhere than at its true site, the nociceptive mechanism is reacting inappropriately. However, since there seems to be logical consistency in the way the errors are made (pain from specific lesions is always referred to the same areas), there must also be a logical explanation for ‘failures’. (If a machine always makes the same mistake, a structural or functional disorder must exist.) The basis for the inadequacy must therefore be sought in a miscalculation in the pain mechanism. Theoretically, the defect can lie anywhere along the afferent pathway, from the peripheral receptors to the synapses in the spinal cord and the reticular area and projection zones in the sensory cortex. • Error at the level of the spinal cord. Most authors have opted for this hypothesis. Mackenzie described an ‘irritable focus’ in the grey matter of the spinal cord as being responsible for the phenomenon.46 Also Livingston47 placed the basis of the error at synapses in the dorsal horns. Wedell et al48,49 and Pomeranz50 accepted a double origin for the sensitive neurone – afferent fibres of a somatic structure, and those coming from a related visceral structure synapse with the same spinal ganglion. Taylor et al51 and Wells et al52 also made a plea for the spinal explanation of referred pain. Their view is that separate peripheral sensory nerves (deep somatic, skin and visceral) converge on to the same cell in the dorsal horn of the spinal cord (Fig. 1.3). • Failure at the sensory cortex. A number of authors have proposed that the misinterpretation is at the projection area of the sensory cortex rather than at the spinal level.33,53,54 The concept was clinically elaborated by Cyriax who based his theory on a number of premises: The ability to localize pain in the region of injury is limited to skin and does not apply when the source of the pain is in deep tissue. In due course, a certain pain memory is built up in the temporal lobes, and achieves a high degree of anatomical precision. The efficacy of the long-term memory storage system is not simply a function of the intensity of the painful experience but also relates to the length of time a painful experience lasts or to the frequency with which it is repeated.55,56 Since the frequency of painful stimuli coming from the skin is much higher than the frequency of stimuli coming from deeper structures, it will be obvious that pain memory will centre around painful experiences from the skin. When the same cortical cells receive a painful message arriving from a deep-seated structure, the memory will interpret it on the basis of past experience; in that the sensory cortex is arranged segmentally, the pain will be ascribed to the correct segment but the system will fail to localize it accurately at the site of the lesion. The brain therefore ‘places’ it in the tissue it has a reference for – the skin. Pain is thus felt under the surface area connected with the particular cells that belong to the same segment as the tissue from which the nociceptive afferents originate. The pain is felt deep to the skin of the relevant dermatome and not accurately in the skin. Before this discussion of referred pain is continued, it should be stressed that root pain reference does not necessarily mean that a nerve is involved. The false idea that wide radiation of pain is evidence of involvement of nerves is still strongly held by some and is usually the most important obstacle to a logical understanding of referred pain. To approach the problem of referred pain with an open mind, the reader must constantly remember that referred pain is an error of perception. Although the nerve supply to these peripheral structures is distributed on a segmental basis, it does not indicate that referred pain ‘runs down’ a somatic nerve. For instance, pain at the anterior aspect of the leg does not necessarily mean that a nerve structure (L3, femoral nerve or peripheral branches of the femoral nerve) is involved. Although inflammation of the dural sleeve of the L3 nerve root does of course lead to pain extending in the L3 dermatome, the same pain can be provoked by a lesion in any other tissue belonging to the L3 segment (e.g. hip joint or psoas bursa). There will not be any difference in the nature and extent of the pain. The only distinction between pain as the result of a compressed and inflamed nerve root and pain originating from trauma to other structures is the appearance of paraesthesia (see Pressure on nerves, Ch. 2). To understand the segmental organization of the nociceptive mechanism, it is necessary to reconsider embryogenesis (Fig. 1.4). When a fetus is between 4 and 6 weeks old, 42 pairs of somites develop: four occipital, eight cervical, 12 thoracic, four to six lumbar, five sacral and eight to 10 coccygeal.57 The first two and the last seven or eight pairs disappear early in development. The ventral aspect of each somite differentiates into the sclerotome which, together with the chorda around the neural tube, forms the origin of the axial skeleton. The other part of the somite becomes the myotome, covered by the dermatome.
Pain
Definition of pain
Perception and modulation of pain
Peripheral nociceptive system
Afferent nociceptive system
Pain modulation systems
Peripheral modulation of pain
Central modulation of pain
At the reticular formation
Referred pain
Introduction
Possible mechanisms
 Referred pain is a pain experience felt elsewhere than at its true site of origin.
Referred pain is a pain experience felt elsewhere than at its true site of origin.
 Skin is an organ adapted to localize the pain accurately.
Skin is an organ adapted to localize the pain accurately.
 Pain is experienced in the sensory cortex, which is organized dermatome by dermatome.
Pain is experienced in the sensory cortex, which is organized dermatome by dermatome.
 The skin is represented accurately in the sensory cortex.
The skin is represented accurately in the sensory cortex.
 A memory storage system is located in the sensory cortex. This is fed by constant input from the skin. Input from deeper somatic structures is very rare in a normal and healthy individual.
A memory storage system is located in the sensory cortex. This is fed by constant input from the skin. Input from deeper somatic structures is very rare in a normal and healthy individual.
Clinical consequences
Rules of referred pain (Box 1.1)
Embryogenesis
Primitive body
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree