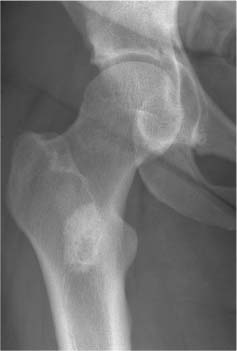
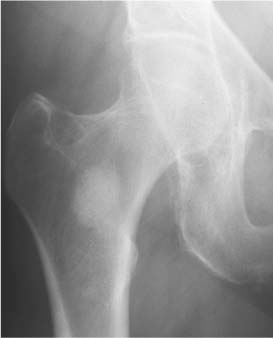
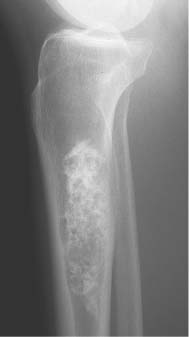
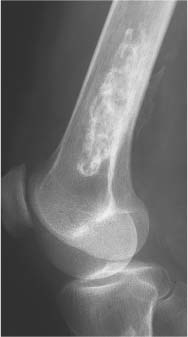
2 Osteosclerosis Osteosclerosis is defined as an increase in bone density caused by increased activity of osteoblasts or by osteogenic or chondrogenic tumor cells forming bone-like tissue. Calcification of tissue other than osteoid within bone is usually dystrophic in nature and may also increase the bone density radiographically. Ossifications within the medullary cavity commonly present as homogeneous, fluffy, cotton-like or cloud-like densities. They most often are caused by bone islands or osteoblastic metastases (Figs. 2.1 and 2.2). Calcifications within the medullary cavity typically present as punctate, annular, comma-shaped or shell-like densities and are commonly associated with chondroid matrix tumors and bone infarcts (Figs. 2.3 and 2.4). The increase in bone density may be scattered or diffuse. This distinction appears useful in the differential diagnosis of osteoblastic reactions, since certain diseases may exclusively present as scattered (solitary or multiple) sclerosis. Accordingly, the differential diagnosis of these entities will be discussed separately in Tables 2.1 and 2.2. Table 2.3 lists sites and commonly used eponyms for idiopathic avascular necrosis. Fig. 2.1 Bone island. A sclerotic focus is seen in the intertrochanteric area. The lesion depicts both tiny radiating bone spicules in its periphery and a central radiolucency, both radiographic features that help to differentiate it from an osteoblastic metastasis. Fig. 2.2 Osteoblastic metastasis (breast carcinoma). An osteoblastic lesion is seen in the intertrochanteric area. Fig. 2.3 Enchondroma. An oblong lesion consisting of multiple irregular, often punctate calcifications is seen in the proximal tibia shaft. Fig. 2.4 Bone infarct. An oblong radiodense lesion with shell-like calcifications is seen in the distal femur shaft. Table 2.1 Solitary or Multiple Scattered Osteosclerotic Lesions
Disease | Radiographic Findings | Comments |
Bone island (enostosis) (Fig. 2.5) | Well-circumscribed isolated area of increased density rarely exceeding 1 cm in diameter. A very slow growth in size is occasionally observed. Margins demonstrate characteristically tiny spiculations or a “brush” border. A central radiolucency is occasionally observed. Occur at any location but pelvis and upper femora appear to be most common locations. | Radionuclide bone imaging is normal. (DD: Osteoblastic metastases are invariably associated with a markedly increased radionuclide uptake.) A large, very dense and structureless bone island within the medullary cavity is often called enostoma (Fig. 2.6). Without proper clinical history such a lesion is often indistinguishable from a surgically excised and methylmethacrylate cemented bone lesion (Fig. 2.7). |
Osteopoikilosis (Fig. 2.8) | Multiple round or ovoid bone densities ranging in size from 2 mm to 2 cm. May demonstrate a radiolucent center. Can be found in all bones, but skull, mandible, ribs, sternum, and vertebrae are only rarely involved. In long bones they are characteristically located in metaphyses and epiphyses, whereas in the scapula and pelvis they are typically found around the glenoid fossa and acetabulum, respectively. | Rare familial disorder not associated with clinical symptoms and therefore incidentally discovered at any age. No increased radionuclide uptake is found in bone scans. |
Osteopathia striata (Fig. 2.9) | Dense longitudinal striations that involve the metaphyses and may extend into the epiphyses and diaphyses. In the ilium, the linear densities radiate from the acetabulum. Vertebral bodies and skull may also be involved. | Rare and usually asymptomatic bone disorder. Occasionally associated with focal dermal hypoplasia (Goltz’s syndrome) |
Chondrodysplasia punctata (congenital stippled epiphyses) (Fig. 2.10) | Multiple punctate calcifications occurring in the epiphyses before the normal time of appearance of the epiphyseal ossification centers. DD: Zellweger’s cerebrohepatorenal syndrome, where the stippling is limited to the patella. | Rare genetically heterogeneous epiphyseal dysplasia associated with a broad spectrum of clinical symptoms. Affected bones may be shortened, or the disorder may regress and leave no deformities. The epiphyseal calcifications may disappear by the age of 3, or may gradually increase in size and coalesce to form a normal-appearing single ossification center. |
Multiple epiphyseal dysplasia (Fairbank’s disease) | Irregular mottled calcifications of the epiphyses diagnosed in children and adolescents. Sequelae in the adult consist of epiphyseal irregularities, degenerative joint changes, and rarely, asymmetrical shortening of tubular bones. | Can be considered to be the tarda form of chondrodysplasia punctata. Cretinism with delayed appearance of stippled and fragmented epiphyseal ossification centers and sclerotic metaphyseal bands must be differentiated. |
Melorheostosis (Fig. 2.11) | Presents in early stage as linear hyperostosis beginning at one end of a tubular bone, progresses with time towards the diaphyses, and results finally in cortical thickening involving either one side or the entire cortex. The lesion may simulate wax flowing down the side of a candle. Osteoma-like protrusions and soft tissue ossifications may be associated. | Often limited to a single limb, in which one or more bones may be affected. At an advanced stage it is part of the differential diagnosis of diffuse osteosclerosis and will be discussed in Table 2.2. |
Osteoma (Fig. 2.12) | Protruding mass lesion composed of abnormally dense bone with structureless appearance. It rarely exceeds 2 cm in diameter, and is usually confined to bone that is produced by the periosteal membrane. It arises from the outer or inner table of the skull, the paranasal sinuses (especially frontal and ethmoid), from the mandible, maxilla, and rarely from the tubular bones of the extremities. | Benign hamartomatous lesion consisting exclusively of osseous tissue. Gardner’s syndrome: Multiple osteomas associated with soft tissue tumors and pre-malignant polyposis, mainly of the colon. |
Benign and malignant bone tumors (Figs. 2.13, 2.14 and 2.15) | Predominantly sclerotic lesions, either as a solitary focus (enchondroma, osteochondroma, chondrosarcoma, osteoid osteoma, osteoblastoma, osteosarcoma, and Ewing’s sarcoma) or as multiple foci (enchondromatosis or Ollier’s disease, hereditary multiple exostoses or diaphyseal aclasis, and osteosarcomatosis). | Differential diagnosis of bone tumors is discussed in detail in Chapter 5. |
Osteoblastic metastases (Fig. 2.16) | Poorly defined areas of increased density with indistinct or lost trabecular structure. With increase in size, adjacent metastases may coalesce, resulting finally in most diffuse sclerosis. With the exception of renal and most thyroid carcinomas which produce invariably lytic and often expansile metastases, osteoblastic metastases may originate from virtually every carcinoma, but carcinoma of the prostate and breast are the most common sources. Other primary tumors include osteosarcomas, carcinoids, and carcinomas originating in the lung, nasopharynx, gastrointestinal tract and urinary bladder. Of the lymphomas, Hodgkin’s disease and histiocytic lymphoma (reticulum cell sarcoma) are most likely to produce osteoblastic bone lesions. | In children, leukemia, neuroblastoma, and Ewing’s sarcoma metastases must be considered, although these lesions are more commonly lytic before treatment is instituted. |
Multiple myeloma | Focal sclerotic lesions are a rare initial manifestation. | Characteristic lytic lesions may become sclerotic with proper therapy. |
Plasma cell granuloma (Fig, 2.17) | Solitary or scattered osteoblastic foci that are only slowly growing. | Consists histologically of a dense infiltrate of normal plasma cells. In contrast to multiple myeloma and plasmacytoma, all laboratory findings are normal. Nowadays considered to be a variant of chronic recurrent multifocal osteomyelitis (CRMO) or SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis), respectively |
POEMS syndrome | Solitary or multiple osteosclerotic lesions and fluffy spiculated hyperostotic areas preferentially at sites of ligamentous attachment in axial and para-axial locations. | POEMS is the acronym for polyneuropathy, or-ganomegaly, endocrinopathy, M protein, and skin changes. |
Chronic or healed osteomyelitis (Fig. 2.18) | May present as localized, thickened sclerotic bone usually containing radiolucent areas. | Bacterial, tuberculous, and fungal organisms may all cause a chronic sclerosing-type osteomyelitis. Sclerosing osteomyelitis of Garre is a low-grade chronic infection not associated with bone destruction or sequestration. |
Brodie’s abscess (Fig. 2.19) | Central lucency surrounded by a slight to extensive reactive sclerosis. Located typically in the metaphysis and, less commonly in the epiphysis or diaphysis of tubular bones. Cortical thickening, periosteal new bone and sequestrum formation may be found with Brodie’s abscess, but are not typical. | Chronic, often painful lesion. |
Tropical ulcer | Often expansile lesion involving preferentially the lower half of the tibia. Associated with periostitis, resulting in localized fusiform periosteal and cortical thickening or even broad-based excrescences resembling osteomas. | In patients of all ages in Central and East Africa. |
Callus formation (Fig. 2.20) | Healed fractures result in a localized increase in bone density. | Commonly in ribs or metatarsals following a fatigue fracture. |
Stress fracture (Fig. 2.21) | Findings dependent on both location and time. A fracture line (cortical or complete) is not always evident. In the shaft of tubular bones, localized cortical thickening and periosteal reaction are characteristic. At the end of tubular bones (e.g. femoral neck, tibia plateau) and in cancellous bone, a band-like focal sclerosis without appreciable periosteal reaction is usually found. | Presents clinically with activity-related pain that is relieved by rest. Radionuclide examination and magnetic resonance imaging are both much more sensitive for early detection and diagnosis. A stress fracture occurring in normal bone under abnormal (increased) stress is referred to as a fatigue fracture, whereas a stress fracture occurring in abnormal (osteopenic) bone with normal stress is referred to as an insufficiency fracture. |
Healed bone lesion (Fig. 2.22) | Spontaneously or under appropriate treatment, healed lytic lesion may become sclerotic. | Benign bone lesions such as fibrous cortical defects and nonossifying fibromas may spontaneously regress and persist as sclerotic foci. Lytic metastases (e.g., from bronchogenic carcinoma, breast carcinoma, lymphoma) and multiple myeloma manifestations may respond to local irradiation, chemotherapy, and/or hormone therapy by becoming osteosclerotic. Brown tumors in primary hyperparathyroidism become sclerotic after removal of the parathyroid adenoma. |
Bone infarcts (old) (Figs. 2.23 and 2.24) | Most often found in the proximal or distal ends of long tubular bones. Healed infarcts present as irregularly calcified areas in the medullary cavity, demarcated from the normal bone by a dense serpiginous contour or irregular streaks. The calcifications may eventually progress to ossification. | Infarcts are often associated with other diseases such as occlusive vascular disease, sickle cell anemia, pancreatitis, connective tissue disease, caisson disease, Gaucher’s disease, and radiation therapy. A similar calcification in the medulla of long bones can occasionally be seen after removal of an intramedullary rod. Enchondromas can simulate bone infarcts (Fig. 2.25). |
Radiation osteonecrosis (Fig. 2.26) | May present years after therapy as a mixture of sclerotic and lytic lesions even when no infarcts have occurred. | This condition can be differentiated from a local tumor recurrence with bone involvement by a normal or even depressed uptake on a bone scan. |
Avascular (epiphyseal) necrosis (AVN) (Fig. 2.27) | Sequelae of avascular necrosis in epiphyses consist of sclerotic and cystic areas of flattened and irregular joint surfaces, which lead to early secondary degenerative changes, particularly in the weight-bearing joints. Most idiopathic avascular necroses occur during childhood and adolescence. Idiopathic avascular necroses occurring in adulthood are found in the medial femur condyle (Ahlbäck’s disease, also reffered to as spontaneous osteonecrosis about the knee or SONC) and in the lunate (Kienböck’s disease). Ahlbäck’s disease typically occurs in the elderly with female predominance and occasionally affect the lateral femoral ar the tibial condyles. New evidence suggest it represents a stress (insufficiency) fracture rather than a spontaneous osteonecrosis. Kienböck’s disease is usually found in young adults. In an advanced stage, the lunate shows increased bone density, fragmentation, and compression. | May be found in any disorder associated with medullary bone infarcts. Avascular necrosis is caused by interruption of the blood supply to the epiphyses with subsequent death of the hematopoetic cells in 6–12 hours, osteocytes in 12–48 hours, and lipocytes in 2–5 days. The etiology may be traumatic (e. g., femoral neck fracture), thromboembolic (e. g., sickle cell disease), vasculitic (e. g., systemic lupus erythematosus), stereoidal, marrow-infiltrative (e. g., Gaucher’s disease) or idiopathic (e.g., Legg-Calvé-Perthes disease). An idiopathic genesis of this abnormality is commonly associated with an eponym (see Table 2.3). |
Paget’s disease (Fig. 2.28) | Can cause uniform areas of increased bone density in the sclerotic phase. In the reparative (mixed lytic and sclerotic) stage, the disease is characteristically associated with cortical thickening resulting in enlargement of the affected bone. Any bone can be affected; “cotton wool” appearance of the skull and “ivory vertebral body” are representative examples of the sclerotic phase of the disease. | Purely sclerotic phase is less common than the combined destructive and reparative stage virtually pathognomonic for the disease. |
Fibrous dysplasia | Besides having a cyst-like or “ground glass” appearance, it can also present as purely sclerotic lesions. Manifestations are usually associated with bone expansion, particularly in tubular bones. With more extensive involvement, bone deformities almost invariably occur. | Occurs in monostotic and polyostotic forms. An ossifying fibroma of the skull, face and mandible cannot be histologically differentiated from fibrous dysplasia, and can be considered radiographically as a localized manifestation of this disease. Ossifying fibromas occuring in long bones, especially in the tibia and to a lesser extend fibula are referred to as osteofibrous dysplasia (Fig. 2.29) |