Chapter 18 Orthoses for brachial plexus injuries
The number of brachial plexus injuries that occur each year is difficult to ascertain. However, with the advent of more extreme sporting activities, more powerful motor sports, and the increasing number of survivors of high-speed motor vehicle accidents (secondary to the introduction of the airbag), the number of plexus injuries continues to rise in many centers throughout the world.3,6,7,10-,11,12,23,24,32 Demographically, a majority of patients with brachial plexus injury are males between 15 and 25 years of age.2,3,24,44 The type and mechanism of injury to the plexus can effectively be summed up by Narakas’ law of seven seventies.54 Based on his 18+ years of experience with more than 1,000 patients with plexus injuries, Narakas has estimated that 70% of traumatic brachial plexus injuries occur secondary to motor vehicle accidents, and of the vehicle accidents, 70% involve motorcycles or bicycles. Of the cycle riders, 70% have multiple injuries. Overall, 70% have supraclavicular lesions, and of those with supraclavicular lesions, 70% have at least one root avulsed. If patients have a root avulsed, at least 70% of these patients with have avulsions of the lower roots (C7, C8, or T1). Finally, of patients with lower root avulsion, nearly 70% will experience persistent pain.
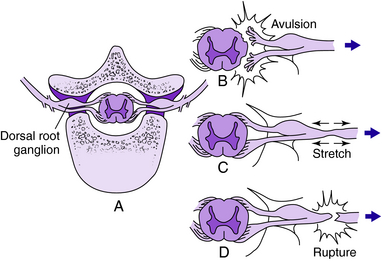
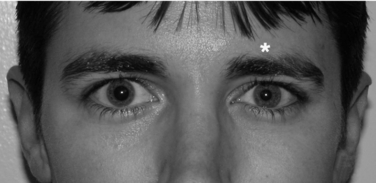
Evaluation of brachial plexus injuries often is performed after the acute life-threatening injuries are treated. Once and if the patient is cooperative, a thorough physical examination should be performed to determine if the lesion is preganglionic (i.e., the root is avulsed directly from the spinal cord proximal to the dorsal root ganglion) or postganglionic (avulsed or injured distal the dorsal root ganglion, within the trunk, divisions, cords, or terminal branches). The differentiation of preganglionic versus postganglionic injury has significant prognostic and therapeutic implications. Preganglionic injury indicates that avulsion of the nerve root has occurred proximal to the spinal root ganglion; complete motor and sensory loss in the involved root and denervation of the deep paraspinal muscles of the neck have occurred (Fig. 18-1). Specific clinical findings are pathognomonic for root avulsions. Rhomboid paralysis is indicative of a C5 avulsion, serratus anterior paralysis is consistent with C5, C6, and C7 avulsion, and Horner syndrome (ptosis, miosis, and anhidrosis) is pathognomonic for C8, T1 avulsions (Fig. 18-2). Postganglionic injuries occur distal to the spinal ganglia and have a more favorable prognosis than do preganglionic injuries both for spontaneous recovery and for surgical reconstruction.
Historically, treatment recommendations for complete root avulsions have varied widely over the past 50 years, and the results of treatment have been fair to dismal. Following World War II, the standard approach was surgical reconstruction by shoulder fusion, elbow bone block, and finger tenodesis.38 In the 1960s, transhumeral (above elbow) amputation combined with shoulder fusion in slight abduction and flexion was advocated.31 The classic paper of Yeoman and Seddon75 noted the tendency to become “one-handed” within 2 years of injury, which led to a dramatic reduction in successful outcomes regardless of the treatment approach. Their retrospective study revealed no “good” results from the primitive surgical reconstruction of that era but predominantly “good” and fair” outcomes when amputation plus shoulder fusion were performed within 24 months of injury. They also noted that the loss of glenohumeral motion caused by brachial plexus injury limited the effectiveness of body-powered devices and that manual laborers seemed to accept hook prostheses much more readily than did office workers with similar injuries. Although these observations remain valid today, recent advances in brachial plexus reconstruction have yielded outcomes superior to historical results. A better understanding of the pathophysiology of nerve injury and repair as well as the recent advances in microsurgical techniques have allowed reliable restoration of elbow flexion in addition to useful prehension of the hand. The current advances in brachial plexus reconstruction, especially the microvascular reconstructive procedures, and the prosthetic/orthotic advances are the focus of this chapter.
Role of surgery
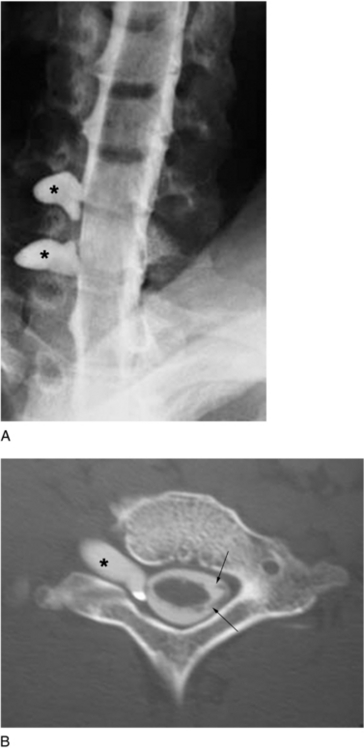
In the most ideal situation, evaluation of the brachial plexus by electrodiagnostic studies should be performed by 3 to 4 weeks after injury, followed by computed tomographic (CT) myelography to evaluate the status of the cervical roots. The role of magnetic resonance imaging in the evaluation of the injured brachial plexus continues to evolve. It is best used for visualizing the dorsal and ventral rootlet but has been less accurate than CT myelography in determining root avulsion.13,25,53 We prefer CT myelography; in our practice, it is the most sensitive and specific test for determining root avulsion injuries (Fig. 18-3). During the early postinjury course, consultation with a pain management team and hand therapist should be initiated as soon as possible to address the severe neuritic pain and prevent joint contractures.43,47,50,51,54,61,62,72–75
Treatment
Several broad categories of surgical treatment of brachial plexus injuries include primary nerve repair, interposition nerve cable grafting, tendon/muscle transfers, neurotization, and free functioning muscle transfers. Tendon and muscle transfers should be delayed until it is evident that further recovery is unlikely. Primary repair is indicated in acute sharp lacerations, whereas interposition nerve cable grafting is indicated in postganglionic injuries less than 6 months old. Neurotization refers to restoration of function by transfer of a functional but less important nerve to the distal but more important denervated nerve and is indicated for preganglionic lesions less than 6 months old. Free functioning muscle transfer refers to transplantation of a muscle and its neurovascular pedicle to a new location and neurotizing of the motor nerve to the flap (Fig. 18-4). Free functioning muscle transfers are indicated for delayed (between 3 and 6 months) or late presentations (>12 months)
Nerve grafts
Sources of donor nerve grafts include the sural nerves, ipsilateral cutaneous nerves, lateral cutaneous nerves of the thigh, saphenous nerve, and ulnar nerve (if C8 and T1 are avulsed). The size mismatch between the plexus and the individual nerve often requires use of multiple strands of nerves bundled together. Often these nerve segments are cabled together with fibrin glue and then sewn in place. In patients with complete brachial plexus avulsions, the ulnar nerve can be harvested as a vascularized nerve graft based on the superior collateral ulnar artery and can be effectively used as a free vascularized interposition nerve graft from neurotization sources to the median nerve (Fig. 18-5).
Neurotization
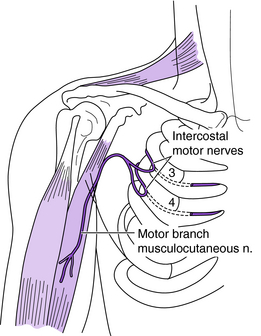
Intercostal nerves can be harvested from the third, fourth, fifth, and sixth ribs and can be effectively used to provide motor nerves to targeted muscles or sensation to injured sensory nerves.15,19,30,37,48,52,66,71,75 Each intercostal nerve has a motor and sensory branch and can be easily harvested and neurotized to the target nerves (Fig. 18-6). Each intercostal nerve contains approximately 1,300 myelinated axons, so typically two to three intercostal nerves are used together. Occasionally the intercostal nerve requires elongation with a nerve graft because the distance to reach the target nerve/muscle is too long. The greatest disadvantage to using a nerve graft is that two lines of coaptation must be crossed. The advantage of using a nerve graft is that the intercostal nerve can be transected more proximally where the number of motor fibers is greater.
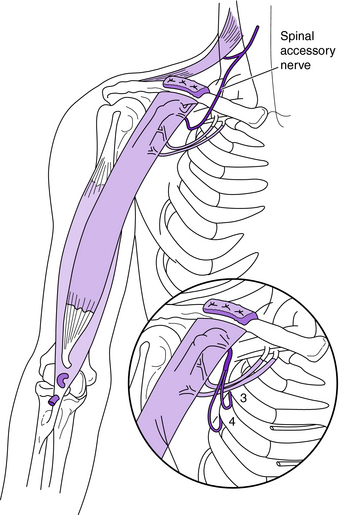
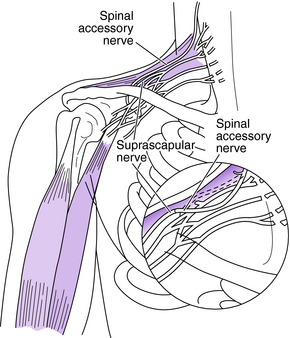
The spinal accessory nerve (cranial nerve XI) also can be used as a donor nerve.4–6,15,16,23,24,27–29,37,48,52,64,66,71 The terminal branch of the spinal accessory nerve can be easily harvested and has approximately 1,700 myelinated axons. It is an excellent donor nerve for the suprascapular or axillary nerve to restore shoulder stability or neurotize a free functioning muscle transfer (Fig. 18-7).
The ipsilateral phrenic nerve can be used; however, prior to its use the diaphragmatic and pulmonary function must be assessed.15,52,66 Hemidiaphragmatic paralysis is an absolute contraindication to phrenic neurotization. Patients with severe chest injury or multiple fractured ribs must be carefully evaluated with respect to pulmonary function because harvest of the phrenic nerve can further jeopardize pulmonary function. Simultaneous intercostal nerve and phrenic nerve harvest results in early postoperative restriction of pulmonary function.35 Phrenic nerve harvest is contraindicated in young infants and in patients with any diaphragm paralysis. The phrenic nerve is best suited for neurotizing the suprascapular nerve.
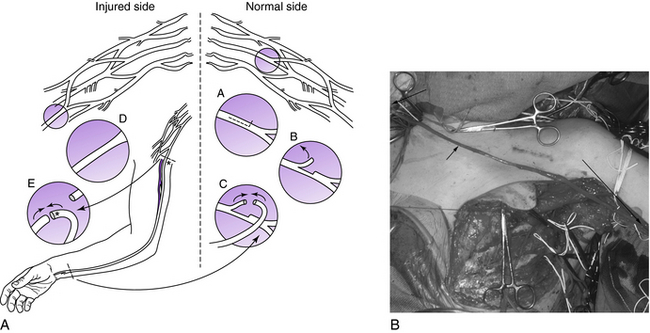
One advance associated with a controversial donor nerve is use of the uninjured contralateral C7 root. Its use was first described by Gu et al.36 in 1992 and then by Chuang et al.18 in 1993. These authors reported that transaction of the uninjured contralateral C7 did not produce significant functional loss. By elongating the C7 root from the contralateral side with a vascularized ulnar nerve graft to the ipsilateral median nerve, the contralateral C7 root can be used to neurotize the median nerve or lateral cord. Use of the anterior superior portion of C7 also has been reported and has lessened morbidity compared to use of the entire contralateral C7 root (Fig. 18-8).68 Donor site morbidity is minimal, initially resulting in digital paresthesias in the C7 distribution and mild weakness of pectoralis, triceps, and/or wrist extension.18,34,68 Over a period of 3 to 6 months, the paresthesias diminish, as does the motor weakness. Typically functional loss is limited to a reduction of sensibility of the index finger and some reduction in force of the triceps and finger extension.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree