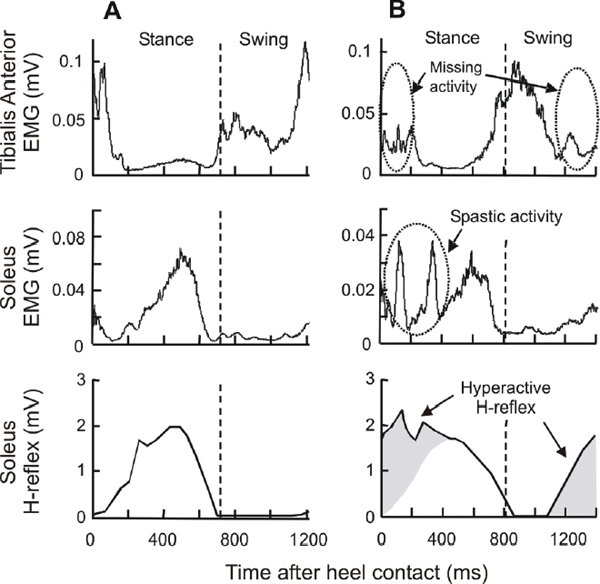
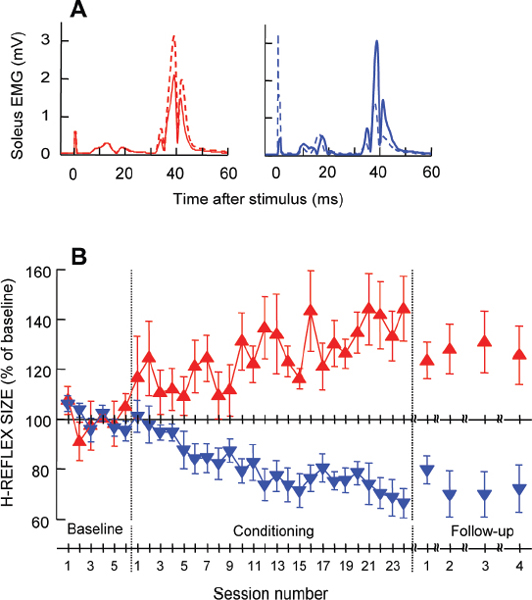
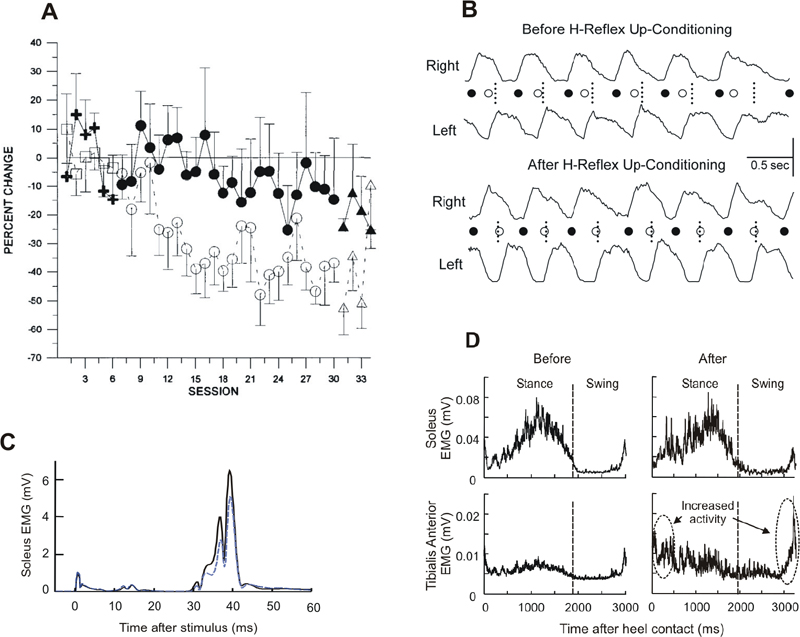
48 Key Points 1. Gradual activity-dependent plasticity shapes spinal cord function during early development and throughout life. 2. Spinal cord injury (SCI) often leads to abnormally functioning spinal reflexes that contribute to impaired movement control. 3. Thus a method that can induce and guide the plasticity in spinal cord pathways may offer a promising new therapeutic approach. 4. Operant conditioning of spinal reflexes induces central nervous system (CNS) multisite plasticity and can improve gait in animals with partial SCI. 5. Operant conditioning of spinal reflexes is possible in people and may improve gait in chronic incomplete SCI. The spinal cord contains neural circuits that are capable of generating movement without direct input from the brain (i.e., reflexes and rhythmic movements) and are plastic throughout life.1 Appropriate appreciation of the spinal cord’s complex capacities for long-term activity-dependent plasticity has occurred only recently.2,3 Methods for inducing and guiding this spinal cord plasticity could help to restore motor functions after spinal cord injuries (SCIs) or other chronic central nervous system (CNS) damage or disease.3 Protocols for operant conditioning of spinal reflexes can be used to produce changes in specific spinal reflex pathways, and they might thereby contribute to the recovery of useful function. The spinal cord receives a continual barrage of descending and peripheral inputs throughout life. In the short term, these inputs produce appropriate movements (e.g., via voluntary muscle activation,4,5 reflex modulation,6–8 etc.). In addition, in the long term, they gradually establish and maintain spinal cord pathways in a state that supports the entire roster of motor behaviors.9,10 Gradual activity-dependent plasticity, driven by descending and associated peripheral inputs, shapes spinal cord function during early development and throughout life.11 Both descending and peripheral inputs have crucial roles in the developmental plasticity that produces a normally functioning adult spinal cord that has characteristic adult reflex patterns and supports motor skills like posture, locomotion, dancing, and playing musical instruments. Disturbances to the descending activity early in life lead to an inappropriately functioning adult spinal cord. During early life, the corticospinal connections important for motor control and skill learning12,13 develop their normal pattern of mainly contralateral innervation of limb muscles.14 However, perinatal supraspinal damage (e.g., cerebral palsy) prevents the normal development of these pathways, and abnormal bilateral projections to the spinal cord may persist into adulthood. Perinatal disruption of descending activity also prevents proper development of the spinal proprioceptive reflexes15,16 that contribute to normal motor behaviors17,18 and results in motor disabilities in the adult. Similarly, properly functioning flexor withdrawal reflexes from noxious stimuli depend on appropriate descending and peripheral inputs during early development,19,20 and descending activity is also crucial to the development of normal urinary function.21 Peripheral and descending inputs are both essential for shaping a properly functioning adult spinal cord. These inputs continue to modify spinal cord pathways throughout adult life. Because SCI disturbs both descending and peripheral activity, it produces motor dysfunction and spinal reflex abnormalities. Normally, spinal reflexes are modulated in functionally appropriate ways that depend on the motor task. For instance, soleus Hoffmann reflex (H-reflex) gain is high during standing, much lower during walking, and even lower during running.22 This task-dependent modulation of H-reflex gain prevents the saturation of motor output and the reflex feedback loop. Non-reciprocal inhibition, which arises largely from Golgi tendon organs and is present during standing, disappears or changes to excitation during some phases of walking.23–25 In walking, the extensor muscles must remain active to support the body weight as long as the limb is loaded, and positive feedback from Golgi tendon organs serves to maintain extensor activity.25–29 In general, reflex modulation across motor tasks appears to contribute to effective task execution.25–27 Injuries to the nervous system often lead to abnormally functioning spinal reflexes. Normal task-dependent modulation of the soleus H-reflex is greatly diminished or absent (or even reversed) in some subjects with incomplete SCI,30,31 probably due to reduced presynaptic inhibition.32 After SCI, normal nonreciprocal Ib inhibition of the soleus by medial gastrocnemius nerve stimulation is absent,33 and recurrent inhibition of the soleus is exaggerated.34 Such losses of appropriate reflex modulation contribute to motor dysfunction. After SCI, altered spinal reflexes may interfere with the already weakened supraspinal control of gait.30,31,35–37 Foot drop (drop and drag of the foot during the swing phase of the gait cycle), one of the most common problems after incomplete SCI, is probably due to changes in both spinal and supraspinal pathways. Ankle dorsiflexion is weakened after SCI due to disruption of the corticospinal connections that normally contribute to ankle dorsiflexion during the swing phase of walking.4,5,38 The remaining dorsiflexion is often further reduced by exaggerated stretch reflex responses from extensor triceps surae muscles.31,39 These reductions in dorsiflexion result in foot drop. During walking in humans, reflexes normally change depending on the phase of the step cycle.22,40,41 However, after SCI, not only task-dependent modulation (see earlier discussion)37,42 but also phase-dependent modulation of the reflex is greatly diminished or absent.30,31,39 In addition, abnormal reciprocal inhibition between the ankle plantar- and dorsiflexors may contribute to exaggerated stretch reflexes or foot drop.35,37,42,43 (Other mechanisms may also contribute to such spastic movement disorders.44) Figure 48.1 shows sole-us H-reflexes, as well as soleus and tibialis anterior (TA) electromyography (EMG), during walking in a normal subject and in a subject with incomplete SCI. In the normal subject (Fig. 48.1A), H-reflex size is greatly modulated over the step cycle: it gradually increases from the beginning of stance, peaks at the end of stance (i.e., at about the same time as the peak sole-us EMG activity), then rapidly decreases, and it stays low or is absent throughout the swing phase. In contrast, in Fig. 48.1B, the soleus H-reflex is suppressed after push-off (as it should be), but it recovers too soon (i.e., in the middle of the swing phase) and stays high through midstance. This probably contributes to the reduced second TA burst from the end of swing to early stance, which produces toe drop at the end of swing. In addition, the abnormally high H-reflex gain during early stance probably underlies the clonic soleus EMG activity seen in this subject (and many people with SCI) during early-middle stance. Such abnormal EMG activation produces instability during the stance-swing transition and in initial foot placement in early stance.31,39 Another common locomotor reflex abnormality is a loss of reflex modulation throughout the step cycle (not shown).30 It has been suggested that such loss of modulation reflects saturation of the reflex loop.31 Fig. 48.1 Soleus and tibialis anterior (TA) electromyographic (EMG) activity and soleus H-reflex size during the step-cycle. The shaded areas in (B) indicate abnormally high reflex gain. (A) In a normal subject, two distinct bursts of TA EMG activity typically occur during the swing phase and the early stance phase: one from the end of stance to early swing and another during the swing-stance transition. Soleus EMG activity gradually increases from heel contact to push-off, then drops down to near zero, and remains low for the entire swing phase. The soleus H-reflex modulation pattern is similar to the soleus EMG pattern. (B) In a subject with chronic SCI, H-reflex modulation is impaired. The H-reflex is suppressed after push-off (i.e., at the end of stance), but it recovers in the middle of the swing phase and stays abnormally high through the midstance. This high soleus reflex gain probably contributes to the abnormally low TA activity during the late swing to early stance phase. In addition, in the presence of the abnormally high reflex gain during early stance, the foot dorsiflexion at heel contact, which stretches the soleus, triggers clonus (indicated by the dotted circle) and produces instability. H-reflex modulation is often impaired in subjects with chronic incomplete SCI, and this abnormality is likely to affect locomotor EMG activity.30,45,46 As Fig. 48.1B illustrates, unsuppressed extensor reflex activity from swing to early stance may counteract ankle dorsiflexion and contribute to foot drop, and high reflex gain in the swing-stance transition may produce clonus that makes the ankle unstable. Thus decreasing extensor reflex gain or restoring its phase-dependent modulation might improve locomotion. In SCI-injured cats in which treadmill training had improved locomotion, the excitatory and inhibitory effects of group I pathways had also changed appropriately.47 This provides evidence for the link between normalizing transmission in spinal reflex pathways and improving locomotion. Thus operant conditioning of spinal reflexes may offer a promising new approach to achieving therapeutic goals. The next sections review the reflex conditioning methodology, its results in normal animals and humans, and its initial applications to animals and humans with partial SCIs. The standard protocol for operant conditioning of spinal reflexes was originally developed in monkeys,48,49 adapted and used extensively in rats,50 and recently tested in mice.51 Although the protocol was first applied to the spinal stretch reflex,49 subsequent work has focused on the H-reflex (and on reciprocal inhibition).48,50,52,54 The rat H-reflex protocol is described here.50,52 The monkey and mouse protocols are very similar.48,51 Rats are chronically implanted with fine-wire EMG electrodes in the soleus muscle and a stimulating cuff on the posterior tibial nerve. The implanted wires connect through a headmount and a flexible tether and commutator to EMG amplifiers and a nerve-cuff simulator. Soleus EMG is monitored continuously (24 h/d) in the freely moving animal. Whenever the absolute value of soleus EMG remains within a specified range for a random varying 2.3 to 2.7 second period, a stimulus through the nerve cuff that is kept just above M-wave threshold elicits the M-wave and the H-reflex. In the course of normal activity, the animal usually provides 2500 to 8000 of these H-reflex trials per day. For the first 10 days, the animal is exposed to the control mode, in which no reward occurs and the H-reflex is simply measured to determine its baseline (i.e., control) value. For the next 50 days, the rat is exposed to the up-conditioning (HRup) or down-conditioning (HRdown) mode, in which a food reward occurs if the H-reflex is above (HRup) or below (HRdown) a criterion value. Background EMG and M-wave amplitude remain constant throughout. Figure 48.2 shows the results of the operant conditioning in rats, monkeys, and mice. In each species, chronic exposure to the up-( Fig. 48.2 (A) Main pathway of the spinal stretch reflex (SSR) and its electrical analog, the H-reflex. Excitation of the Ia spindle afferents (and possibly large group II afferents)90,91 activates the motoneurons innervating the same muscle and its synergists. This activation is largely monosynaptic. If the afferents are excited by muscle stretch, the response is the SSR. If the afferents are excited by an electrical stimulus, the response is the H-reflex. Although the pathway is entirely spinal, it is strongly influenced by descending influence from the brain. (B) Operant up-conditioning and down-conditioning of a spinal reflex in different models. From left, soleus H-reflex in rats, triceps surae H-reflex in monkeys, biceps brachii SSR in monkeys, and triceps surae H-reflex in mice. In general, the time courses and magnitudes of change are similar across different species and muscles.51–53 (Adapted from Wolpaw JR. The complex structure of a simple memory. Trends Neurosci 1997;20(12):588–594; Chen XY, et al. Reflex conditioning: a new strategy for improving motor function after spinal cord injury. Ann N Y Acad Sci 2010;1198(Suppl 1):E12–21; and Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol 2006;96(4):1718–1727.) Spinal reflex conditioning in humans uses a protocol comparable to that developed in animals. The protocol was first applied in humans to the biceps brachii stretch reflex56,57 and more recently has been applied to the human soleus H-reflex.58 The only significant difference from the animal protocol is that humans perform many fewer trials (i.e., only 2 to 5% as many), and these trials are confined to 1-hour sessions three times per week. Nevertheless, the human results are similar to the animal results in the gradual progression and final magnitude of reflex change. The newly developed soleus H-reflex protocol comprises six baseline sessions and 24 conditioning sessions at a pace of three sessions per week, and four follow-up sessions over the next 3 months. Sessions are always held at the same time of day to control for diurnal variation in the reflex.59 In each session, the soleus H-reflex is elicited while the subject maintains a natural standing posture and a specified stable level of soleus background EMG. The size of the M-wave is kept constant within and across the sessions. In each baseline session, three blocks of 75 control H-reflexes (i.e., 225 H-reflexes) are elicited. In each conditioning or follow-up session, 20 control H-reflexes are elicited as in the baseline sessions, and then three blocks of 75 (i.e., 225) conditioned H-reflexes are elicited. In these conditioned H-reflex trials, the subject is asked to increase (HRup mode) or decrease (HRdown mode) the H-reflex and is given visual feedback after each stimulus to indicate whether the resulting H-reflex was larger (HRup) or smaller (HRdown) than a criterion value. Good performance in changing the reflex size earns an additional monetary reward. Background EMG and M-wave size are kept stable throughout data collection. Figure 48.3 summarizes the human soleus H-reflex conditioning results. Over the 24 conditioning sessions, H-reflex size gradually increased in six of eight HRup subjects and decreased in eight of nine HRdown subjects, resulting in final sizes of 140(± 12 standard error of mean [SEM])% and 69(± 6)% of baseline size, respectively. In these subjects, the final H-reflex change was the sum of within-session change (i.e., task-dependent adaptation) and across-session (i.e., long-term) change. Task-dependent adaptation appeared within four to six sessions and persisted thereafter, whereas long-term change began after 10 to 12 sessions and increased gradually thereafter. (Full presentation and discussion of task-dependent adaptation and long-term change can be found elsewhere.58) This study showed that people performing only 225 reflex conditioning trials a day, 3 days a week, displayed gradual reflex change similar in course and nearly equal in magnitude to that of animals that perform 20 to 50 times as many trials. This indicates that H-reflex conditioning is possible in humans and does not require the several thousand trials per day typically completed by animals. (Animals probably do not require that many trials either, but that remains to be determined.) The success rate of 82% (14 of 17 subjects changed H-reflex size significantly in the correct direction) was also comparable to that of animals.48,50,52,58 In addition, this study showed that exposure to the reflex operant conditioning paradigm over several months induced both short-term adaptation and long-term plasticity in spinal reflex pathways. The long-term plasticity appeared to be a lasting change in the reflex pathway that persisted outside of the reflex conditioning paradigm and lasted for at least several months after cessation of reflex conditioning.58 This suggests that it might be possible to use reflex conditioning protocols to guide such long-term plasticity so as to essentially reeducate abnormally functioning spinal reflex pathways and thereby alleviate motor disabilities associated with partial spinal cord injuries (Fig. 48.1). This possibility is supported by a recent study in rats with SCI.60 Fig. 48.3 Human subjects can learn to change H-reflex size in response to an operant conditioning protocol. (A) Average H-reflexes from two representative subjects for a baseline session (solid) and for the last conditioning session (dashed). After the 24 conditioning sessions, H-reflex size is larger in the up-conditioning (HRup) subject (left) and smaller in the down-conditioning (HRdown) subject (right). (B) Average H-reflexes (± SEM) for six successful HRup and eight successful HRdown subjects for baseline, conditioning, and follow-up (12, 30, 60, and 90 days after the end of conditioning) sessions. As in animals, H-reflex size gradually increases in the HRup group (upward triangles) and decreases in the HRdown group (downward triangles) over the course of the study. (With permission from Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 2009;29(18):5784–5792.) Spinal cord reflexes commonly function as parts of complex behaviors, such as locomotion.6–8,41,61–64 At the same, these reflexes are themselves simple behaviors, and operantly conditioned changes in them are essentially simple skills (i.e., “adaptive behaviors acquired through practice” according to the Compact Oxford English Dictionary, 1993). Thus, operant conditioning of spinal reflexes can provide excellent models for studying the plasticity underlying motor skill learning.11,12,52,65 An ongoing series of physiological and anatomical studies has begun to reveal the complex pattern of spinal and supraspinal plasticity underlying H-reflex conditioning.11,12,52 A positive shift in motoneuron firing threshold (possibly due to a change in the activation voltage of Na+ channels) can largely account for down-conditioning of the H-reflex.52 Down-conditioning is also associated with marked increases in identifiable gamma aminobutyric acid (GABA)-ergic interneurons in the ventral horn and GABAergic terminals on the soleus motoneuron.65 There is also evidence that changes occur in several other synaptic populations on the motoneuron, in motor unit properties, in other spinal interneurons, and even on the contralateral side of the spinal cord.52 Up-conditioning and down-conditioning appear to have different mechanisms; they are not mirror images of each other. Up-conditioning may be due to plasticity in spinal interneurons.65 The corticospinal tract (CST) is the only major descending tract that is essential for H-reflex conditioning.66–68 Thus it is presumably CST activity that changes the spinal cord. Furthermore, cerebellar–cortical connections appear to be essential for establishing and maintaining supraspinal plasticity that in turn establishes and maintains the spinal cord plasticity that is directly responsible for H-reflex change.69,70 In sum, the data indicate that H-reflex conditioning depends on a hierarchy in which plasticity in the brain induces plasticity in the spinal cord.58,67–71 Operant conditioning of a spinal reflex produces multisite plasticity that extends far beyond the reflex pathway conditioned and can therefore have complex effects on motor function. Operant conditioning is a powerful method for inducing changes in specific spinal pathways. Because abnormally functioning spinal reflexes contribute to movement disabilities (Fig. 48.1), methods for reducing reflex abnormalities may help to reduce motor disabilities. Segal and Wolf57 showed that it is possible to operantly condition the biceps brachii stretch reflex in humans with incomplete SCI (Fig. 48.4A). In this initial study, they did not address the question of whether such conditioning can produce therapeutic benefits. Chen et al.60 recently showed that up-conditioning of the soleus H-reflex can improve locomotion in rats with incomplete SCI (Fig. 48.4B). Midthoracic hemisection of the right lateral column shortens the right stance phase and thereby produces an asymmetry in locomotion. As Fig. 48.4B illustrates, H-reflex up-conditioning, which enhances the soleus burst during the stance phase, eliminates this asymmetry. This study suggests that reflex conditioning protocols might improve motor function in people with partial SCI. The initial soleus H-reflex conditioning study in normal humans showed that exposure to the operant conditioning paradigm three times per week over several months induced both short-term adaptation and long-term plasticity in the reflex (see earlier discussion).58 If such conditioning is possible in people with partial SCI, it could be used to guide long-term plasticity that reduces specific motor disabilities. To evaluate the therapeutic possibilities of this approach, we have begun to apply a soleus H-reflex down-conditioning protocol in people with spastic gait due to incomplete SCI.72 The initial subjects are adults with chronic (0.7 to 9 years postinjury) incomplete SCI who suffer from ankle extensor spasticity and foot drop. All are medically stable and ambulatory. The protocol is the same as the one used in normal subjects,58 except for an increase in the number of conditioning sessions from 24 to 30. Six baseline and 30 down-conditioning sessions occur at the rate of three sessions per week for 12 weeks. Soleus and tibialis anterior background EMG and soleus M-wave size are maintained at constant levels throughout the study. The initial results indicate that operant down-conditioning of the soleus H-reflex is possible in people after incomplete SCI. Three of the four subjects studied to date had significantly smaller H-reflexes after down-conditioning. Fig. 48.4C shows the before and after results for one of these subjects. Chen et al.73,74 found that the success rate for reflex conditioning in rats with SCI was inversely correlated with the severity of the injury. In rats, the CST is essential for reflex conditioning,67,68 and initial results from people with strokes suggest that the CST may also be important for conditioning in humans.75 Thus incomplete SCIs that involve the CST may impair reflex conditioning. Nevertheless, the study of Segal and Wolf57 and these new results indicate that conditioning is possible in many people with SCI, though it may take longer. After successfully decreasing the sole-us H-reflex, some of the subjects showed changes in locomotor EMG activity. Figure 48.4D shows soleus and TA locomotor EMG in a spastic subject before and after successful down-conditioning of the soleus H-reflex. Before conditioning, soleus EMG was comparable to that found in normal subjects (Fig. 48.1), but TA activity was much lower than normal, and this resulted in foot drop. After successful conditioning, the soleus burst was not noticeably different, whereas TA EMG had increased, especially during the swing-stance transition (i.e., the second burst of the swing-phase TA activity; compare with the normal subject in Fig. 48.1A). This increase in TA EMG served to reduce foot drop, although it was not enough to eliminate it completely. This subject also showed an increase in walking speed (i.e., reduction in 10 meter walk time from 54 to 24 seconds). The two other subjects in whom conditioning was successful also showed improvements in walking speed (15 to 55% reductions in 10 meter walking times). Whether such changes are specific to successful conditioning (and to the direction of conditioning), and how they are related to changes in gait kinematics, reflex modulation over the step-cycle, and other measures of spasticity remain to be determined. Fig. 48.4 (A) Average biceps brachii spinal stretch reflexes (± SEM) over 34 sessions (i.e., six baseline, 24 conditioning, and four follow-up sessions) in people with incomplete spinal cord injury (SCI) who were (?/?) or were not (+/?) exposed (i.e., the control group) to the down-conditioning protocol. In the down-conditioning group, spinal stretch reflex (SSR) size declines steadily over the conditioning sessions and remains low in the follow-up sessions. In contrast, SSR shows only a small insignificant decrease in the control group. (B) The effects of H-reflex up-conditioning on locomotion in a rat with midthoracic right lateral column transection. The traces show the electromyographic (EMG) bursts from right and left soleus muscles during treadmill locomotion before (top) and after (bottom) H-reflex up-conditioning has increased the size of the right soleus H-reflex. The presumed onsets of the right (?) and left (?) stance phases of locomotion are indicated in the middle. The short vertical dashed lines mark the midpoints between right burst onsets, where the left burst onsets should occur. Before H-reflex up-conditioning, the left burst onset occurs too early, and the gait is asymmetrical. H-reflex up-conditioning strengthens the right soleus EMG burst and corrects the left burst onset timing, and thereby reduces the gait asymmetry. Horizontal scale bar: 0.5 second vertical scale bar: 100 and 150 µV for the right and left EMG bursts, respectively. (C) Average H-reflexes in a representative subject with SCI for a baseline session (solid) and for the final conditioning session (dashed) (all 225 trials averaged for each trace). The final H-reflex is substantially smaller than the baseline H-reflex, whereas the background electromyographic (EMG) and M-wave size have not changed. (D) Soleus and tibialis anterior (TA) EMG activity during walking before and after soleus H-reflex down-conditioning in the same subject. Before H-reflex conditioning, there is almost no TA EMG activity throughout the step cycle. After down-conditioning, there is little change in soleus EMG, whereas TA EMG increases, especially in the late-swing to early-stance period. Increased TA activity in this period reduces foot-drop. ([A] from Segal RL, Wolf SL. Operant conditioning of spinal stretch reflexes in patients with spinal cord injuries. Exp Neurol 1994;130(2):202–213. [B] from Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 2006;26(48):12537–12543. Reprinted with permission.)
Operant Conditioning of Spinal Reflexes to Improve Motor Function after Spinal Cord Injury
 Activity-Dependent Spinal Cord Plasticity
Activity-Dependent Spinal Cord Plasticity
 Developmental Plasticity of Spinal Cord
Developmental Plasticity of Spinal Cord
 Abnormally Functioning Spinal Reflexes in Motor Dysfunction after Spinal Cord Injury
Abnormally Functioning Spinal Reflexes in Motor Dysfunction after Spinal Cord Injury
Possible Therapeutic Benefits of Using Operant Conditioning to Induce and Guide Spinal Reflex Plasticity
 Operant Conditioning of Spinal Reflexes in Normal Animals and Humans
Operant Conditioning of Spinal Reflexes in Normal Animals and Humans
Operant Conditioning of Spinal Reflexes in Laboratory Animals
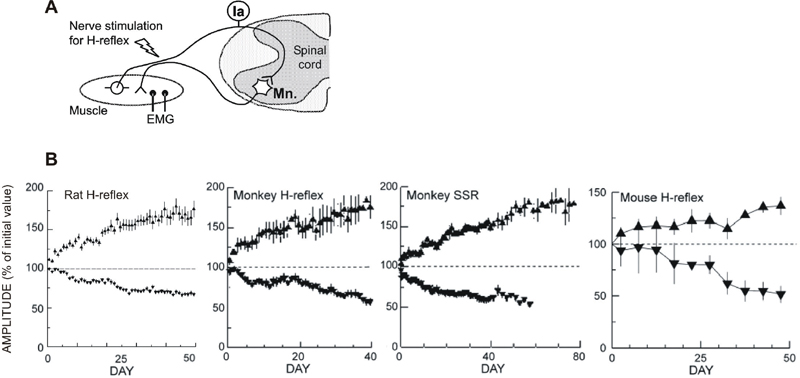
![]() ) or down-(
) or down-(![]() ) conditioning mode gradually changes the size of the reflex in the correct direction. Successful conditioning, defined as a change of > 20% in the correct direction,50,55 occurs in 75 to 80% of the animals. In the remainder, the reflex remains within 20% of its control value.
) conditioning mode gradually changes the size of the reflex in the correct direction. Successful conditioning, defined as a change of > 20% in the correct direction,50,55 occurs in 75 to 80% of the animals. In the remainder, the reflex remains within 20% of its control value.
Operant Conditioning of the Soleus H-reflex in Normal Humans
Current Understanding of the Spinal and Supraspinal Mechanisms of Reflex Conditioning
 Operant Conditioning of Spinal Reflexes after Spinal Cord Injuries
Operant Conditioning of Spinal Reflexes after Spinal Cord Injuries
Operant Conditioning of the Soleus H-reflex in Incomplete Spinal Cord Injury
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
