A 54-year-old female patient was admitted to our outpatient clinic with the complaint of bilateral knee pain, morning stiffness, and decreased walking distance. The diagnosis was bilateral grade 2 osteoarthritis. Activity modification, weight loss, and an NSAID were prescribed. After 3 weeks, the patient returned for a control visit. She was happy with the pain control in her knees with the increase in walking distance, but she had been admitted to the emergency department with stomach pain on the 10th day of treatment. Gastroscopy (endoscopy) revealed hemorrhagic ulcers of the gut. Treatment was initiated with proton pump inhibitors (PPIs). We changed our treatment to viscosupplementation therapy.
Nonsteroidal drugs have a wide spectrum of effects on metabolism that can have some relevant consequences on respiratory, gastrointestinal (GI), renal, hematological, and bone metabolism [1]. Use of these drugs as pain killers is accompanied by the risk of side effects in these systems. The most frequent complication is GI intolerance, which can have dangerous consequences such as fatal gastrointestinal bleeding. Countermeasures should be taken by prophylaxis with PPI drugs.
Introduction
NSAIDs show their effect through inhibition of the cyclooxygenase (COX) enzyme [2]. The COX enzyme provides arachidonic enzyme metabolism and therefore prostaglandin (Fig. 41.1 ) and thromboxane synthesis. The analgesic and anti-inflammatory effects of NSAIDs occur by inhibition of prostaglandin synthesis [3]. Prostaglandins have little effect on pain; other mediators of inflammation can cause pain. Although NSAIDs do not affect other mediators of the inflammatory response, they cause a decrease in inflammation. All NSAIDs have different COX inhibition mechanisms, and their analgesic, antipyretic, and anti-inflammatory effects in high doses are different from one another [4].
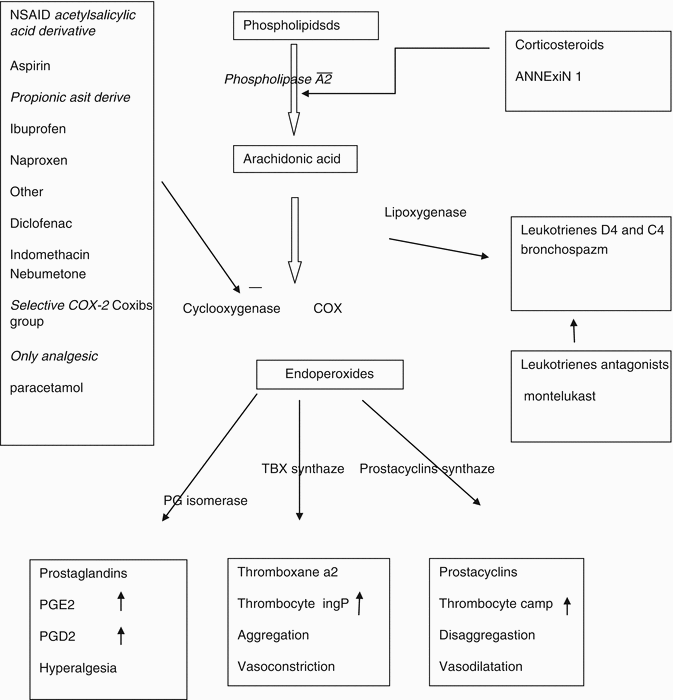

Fig. 41.1
Mechanism of action of NSAIDs and corticosteroids in phospholipid and prostaglandin metabolism
Anti-inflammatory Effect
NSAIDs inhibit the activation of neutrophil leucocytes by the effects that cause inflammation and accompanying events (such as selectin and integrin up-regulation, adhesion to the microvascular wall, and extravasation). They reduce the synthesis of prostaglandins (PGE2, PGI2), which cause vasodilation and edema. They inhibit the activation of polymorphonuclear leukocytes by various stimuli. NSAIDs bind to and inactivate active oxygen radicals and reduce inflammation. They stabilize the lysosome membrane. Because NSAIDs are acidic drugs, they can easily enter into the cells in inflammation and gather there [5, 6].
Analgesic Effect
NSAIDs act by inhibiting the synthesis of prostacyclin and prostaglandin (especially PGE2), which do not create pain alone but increase the awareness of nerve endings to algesic factors (bradykinin, histamine, serotonin, substance P, and angiotensin) and strengthen the pain constructive effects. In addition to inhibiting cyclooxygenase peripherally in their analgesic effects, some NSAIDs (such as dipyrone) play a role in inhibiting in the central nervous system [7] (Table 41.1).
Table 41.1
Physiological and pathological effects of prostaglandins
Functions of prostaglandins | |
|---|---|
Pathological effects | Physiological effects |
Fever | Fever control |
Myocardium | Bronchial tonus |
Asthma | Blood pressure |
Thrombosis | Gastric mucosa |
Ulcers | Renal function |
Diarrhea | Bowel movement |
Dysmenorrhea | Semen viability |
Pain | Cell protector |
Inflammation | |
Bone degeneration | |
Antipyretic Effect
Pyrogenic cytokines, released by inflammatory cells that are stimulated by bacterial toxins (interleukin (IL)-1 and tumor necrosis factor (TNF)-alpha), depend on the blocking effect in the thermoregulatory center via prostaglandins in the hypothalamus. They thereby reduce fever by increasing heat loss. They do not affect normal temperature and hypothermia. It has been found that PGE-2 plays a role in the endogenous fever formation mechanism. Pyojenic cytokines are produced by the enzyme COX-2 and cause fever with the enzyme IL-1. It is suggested that inhibition of the COX-2 enzyme would have a greater antipyretic effect [8, 9].
NSAIDs and COX Inhibition
In the amino acid sequence of the COX-2 enzyme there is valine, which is a smaller amino acid, instead of isoleucine (COX-1) at the 120th position. The COX-2 enzyme has a small gap in the inner surface of the enzyme. The ability to connect to this gap determines a drug’s COX-2 specificity. The two isoenzymes of COX (COX-1 and COX-2) are responsible for the side effects in the gastrointestinal system (GIS) and NSAIDs’ anti-inflammatory effects. The COX-1 isoenzyme is present in many tissues. It allows the formation of homeostatic and cytoprotective prostanoids, which are responsible for physiological functions in organs such as the kidneys and platelets [2, 10]. Well-known side effects of NSAIDs appear with the inhibition of this enzyme. The COX-2 enzyme is induced only by simulation. Synthesis of proinflammatory PG in leukocytes, vascular smooth muscle cells, human rheumatoid synoviocytes, and brain neurons is stimulated with cytokines, mitogens, and endotoxins. The analgesic anti-inflammatory activity of COX-2 is the same as with the classic NSAID, but there are no gastrointestinal, platelet, or renal side effects. However, the effect of the COX-2 inhibitor on fracture healing is different.
NSAIDs have COX-1/COX-2 inhibition rates that have been determined by many different laboratories. Many NSAIDs (nonselective NSAIDs) such as aspirin, naproxen, indomethacin, tolmetin, piroxicam, and ibuprofen cause inhibition in different ratios in the two isoenzymes. It is accepted that COX-1 inhibition of NSAIDs such as etodolac and meloxicam (COX-2 selective) is less than with COX-2 and it is also accepted that COX-2-specific NSAIDs do not effectively inhibit COX-1 [11]. COX-2 is inhibited most powerfully by meloxicam. The GIS side effects are significantly lower compared with classic NSAIDs. Thus, the use of COX-2-specific NSAIDs, which came into use because it was thought that they had no side effects on the stomach, are today discussed and restricted because of the uncertainties of cardiac side effects [4]. Studies show that the COX-2 enzyme inhibits 100.054 more than COX-1 enzyme. NSAIDs that inhibit COX-1 also show an antiaggregant, or antiplatelet, effect with the inhibition of COX-1, which is the only isoform in the platelets. Aspirin acetylates platelet COX-1 irreversibly and, thus, the antiaggregant effect lasts up to 4–6 days until new platelets are made by bone marrow. With other NSAIDs, the inhibition of platelet aggregation is reversible and dependent on the concentration of a drug in the platelets [12]. The antiaggregant effect of aspirin is the reason for its popular use in cardiovascular prophylaxis. But it is also shown that if aspirin is taken with other NSAIDs, there may be an interaction in terms of the antiaggregant effect. The effects of NSAIDs on cartilage have been under discussion for years. Although some animal experiments have shown that NSAIDs may have negative effects on cartilage, there is no clear information about the effects on humans [13].
NSAID Clinical Uses
NSAIDs implement analgesia with both regional and central effects in clinical and soft tissue injuries. NSAIDs are commonly used in the treatment of such diseases as rheumatoid arthritis, gouty arthritis, ankylosing spondylitis, systemic lupus erythematosus and other connective tissue disease, juvenile chronic arthritis, and osteoarthritis because of their anti-inflammatory and analgesic effects. It is also chosen for treatment of soft tissue lesions such as tendinitis, bursitis, tenosynovitis, and periarteritis; back, neck, and cancer pain; dysmenorrhea; and oral and maxillofacial surgery [14]. It is thought that, in the future, after the side effect profile gains clarity, particularly COX-2-specific (coxibs group) drugs will be used in the prophylaxis of malignancies such as colorectal and prostate cancers and Alzheimer’s disease. The most commonly used NSAIDs and their characteristics are listed in Table 41.2.
Table 41.2
Comparison of the therapeutic effects of NSAIDs and steroids with narcotics
Analgesic | Anti-inflammatory | Antipyretic | Local | |
|---|---|---|---|---|
Narcotics | +++ | − | − | − |
Glucocorticoids | + | +++ | − | + |
NSAID | ++ | ++ | +/− | + |
NSAIDs affect healing negatively by inhibiting prostaglandin synthesis in fracture healing (Table 41.3).
Salicylates
Although many drugs are in use, aspirin is still the most widely used analgesic, antipyretic, and anti-inflammatory drug. Salicylates often act through the salicylic acid content in them. Aspirin is used for low-intensity pains such as myalgia headache and arthralgia, and it effectively reduces increased body temperature. Low doses of aspirin (<100 mg) are widely used for cardioprotective effects. If healthy people use aspirin, it prolongs their bleeding time. A single 325-mg dose of aspirin extends a normal individual’s bleeding time by 2 times, for a period of 4–7 days. Thrombocytes reduce COX’s irreversible acetylation and consequently the formation of thromboxane A2 until a sufficient number of unmodified thrombocytes is formed from the megakaryocytes’ precursors [15]. If possible, aspirin therapy should be discontinued 7 days before surgery. Aspirin use should be avoided in patients with severe liver disease, hypoprothrombinemia, vitamin K deficiency, and hemophilia [16]. There is no specific antidote to salicylate poisoning. Treatment should start with rapid assessment and medical emergency intervention.
Para-aminophenol Types (Paracetamol)
Paracetamol is an effective alternative to aspirin as an antipyretic, analgesic agent; however, its anti-inflammatory effect is very low [17]. It is effective as a painkiller in noninflammatory osteoarthritis but cannot be used in place of aspirin or other NSAIDs in chronic inflammatory diseases such as rheumatoid arthritis. Paracetamol is well tolerated and the incidence of GI side effects is low. Acute overdose can cause serious liver damage. Chronic use of less than 2 g per day does not cause liver dysfunction [18] (Table 41.4).
Table 41.3
NSAIDs and their effects
1. Those with analgesic effects and low grades of anti-inflammatory effects |
(a) Aniline derivatives: paracetamol (acetaminophen) |
Analgesic antipyretic – but there are no antithrombotic and anti-inflammatory effects |
2. Those with analgesic effects and low-to-moderate intensity of anti-inflammatory effects |
(a) Propionic acid derivatives: ibuprofen, fenoprofen, ketoprofen, naproxen |
Analgesic anti-inflammatory effect |
(b) Fenamic acid derivatives: mefenamic acid |
3. Those with analgesic effects and high anti-inflammatory effects |
(a) Salicylic acid derivatives: aspirin, sodium salicylate |
(b) Pyrazolone derivatives: propyphenazone, aminopyrine, metamizol, phenylbutazone |
Analgesic, antipyretic, antispasmodic effect, no anti-inflammatory effect |
(c) Phenylacetic acid derivatives: diclofenac, etodolac, fenclofenac |
Strong anti-inflammatory analgesic |
(d) Indole derivatives: indomethacin, sulindac, tolmetin |
More anti-inflammatory and antipyretic than aspirin |
(e) Oxicam derivatives: piroxicam, meloxicam, tenoxicam |
Long-acting analgesic |
4. COX-2 Inhibitors: celecoxib, rofecoxib and parecoxib |
Usage is controversial |
Table 41.4
Studies on the effects of NSAIDs on fracture healing
Negative effects on fracture healing | No retarding effect on fracture healing |
|---|---|
Altaian RD, J Orthop Trauma 1995 | Adolphson P, Arch Orthop Trauma Surg 1993 |
Beck A, Arch Orthop Trauma Surg 2003 | Bichara J, J Perodontol 1993 |
Giordano V, Injury 2003 | Bragger IL, J Perodontal Res 1997 |
Glassman S, Spine 1998 | Moore KD, J Bone Joint Surg Br 1998 |
Ho M, Pharmacology 1998 |
Acetic Acid Derivatives (Indomethacin, Sulindac, and Etodolac)
High rates of intolerance restrict the long-term use of indomethacin as an analgesic. Indomethacin successfully removes joint pain, swelling, and tenderness; improves grip strength; and reduces the duration of morning stiffness [14]. Typical usage is 25 mg, two to three times a day. It is more effective than aspirin in the treatment of osteoarthritis and ankylosing spondylitis, if well tolerated. It is approved by the US Food and Drug Administration (FDA) in the closure of patent ductus arteriosus [19].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








