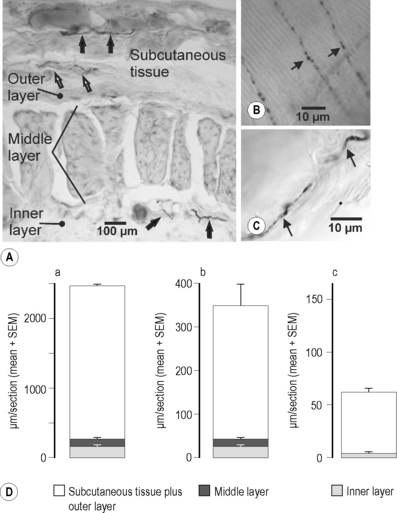
2.4 Nociception In the literature, the thoracolumbar fascia (TLF) is usually assumed to have a mechanical function connecting the latissimus dorsi muscle as well as the abdominal muscles to the spine and iliac crest. It continues craniad up to the skull and caudad to the fascia of the lower extremity. Actually, it connects the latissimus dorsi muscle to the gluteal muscles, thus functionally linking the arm with the leg. Other functions are: (1) forming a sheath around muscles that reduces friction during movements, (2) facilitating the return of venous blood to the heart, (3) providing an ectoskeleton for the attachment of muscles, and (4) protecting blood vessels and muscles from mechanical damage (e.g., the lacertus fibrosus of the biceps brachii muscle or the aponeuroses of the palm or soles (Benjamin 2009). Recent data indicate that fascia in general is not just a passive structure but is contractile. The basis of the contractility is myofibroblasts that appear to be present in many fascia and perform very slow “contractions” lasting many minutes when the tissue is stimulated chemically in vitro (Schleip et al. 2007). In the opinion of many clinicians this finding is trivial, because the high prevalence of the Dupuytren-syndrome shows that the palmar aponeurosis has just this capability, although it is not clear if the mechanism of the Dupuytren contracture is dependent on myofibroblasts. The fascia has also been assumed to be involved in acupuncture effects, in that planes of connective tissue have a close relation to acupuncture points and react very sensitively to the rotations of acupuncture needles (Langevin et al. 2007). Finally, the fascia has been discussed as a possible source of pain in patients with non-specific low back pain (Yahia et al. 1992). This type of back pain does not originate in bony structures of the spine or the facet joints but in the soft tissues of the low back (muscles, ligaments, fasciae). Non-specific low back pain is one of the most common pain complaints in industrialized countries; therefore, the clarification of a possible contribution of fascia receptors to this pain would be of importance not only for our understanding but also for the management of this type of pain. To fulfill the role of a pain source in non-specific low back pain, the fascia should have a dense innervation with sensory fibers. However, the TLF was largely ignored as a subject of scientific studies and, therefore, little information is available on the innervation of the TLF and hence on the possible sensory role of the fascia. Even recently, Panjabi (2006) published a comprehensive report of mechanisms of pain generation in non-specific low back pain, but did not mention the TLF as a potential source. Interestingly, the innervation of other structures, such as the small ligaments and the intervertebral discs of the spine, was studied as early as the sixties with the histological methods available at that time (methylene blue; Hirsch et al. 1963). Stecco and colleagues (2007) likewise found abundant nerve fibers in the fasciae of the upper limb, including retinacula and the lacertus fibrosus. Another problem in assessing the role of the TLF in low back pain is that the few studies performed earlier on fascial innervation are partly contradictory, or questionable with regard to the conclusions drawn by the authors. For instance, Bednar and colleagues (1995) stated that the TLF of low back pain patients “is deficiently innervated”. The reason for this conclusion is that the authors did not find any sensory receptors in the tissue specimens they studied. However, the results of a histological study on human specimens by Yahia and colleagues (1992) did show that the TLF is innervated and exhibits free and encapsulated nerve endings. The encapsulated nerve endings probably represented mechanoreceptors. For this article, the free nerve endings are more interesting, because many of them are nociceptive and subserve pain. For the tibial anterior fascia, an important role in the pain of delayed onset muscle soreness (DOMS) has been suggested in a study on human subjects (Gibson et al. 2009). In this investigation, hypertonic saline was injected as a pain-producing agent into the muscle and the overlying structures after induction of DOMS in that muscle. The main result was that the injection directly underneath the fascia caused more pain than into the muscle itself. • A thin outer layer consisting of parallel collagen fibers oriented transversely (in the coronal plane). • A thick middle layer composed of massive collagen fiber bundles running obliquely to the long axis of the body. • A thin inner layer consisting of loose connective tissue covering the underlying multifidus muscle (Fig. 2.4.1A). All fibers were visualized with immunohistochemical techniques. As a universal marker for all nerve fibers, antibodies to protein gene product 9.5 (PGP 9.5) were used (Lundberg et al. 1988). The coronal section in Fig. 2.4.1(A) shows PGP 9.5-immunoreactive (ir) neuronal structures mainly in the subcutaneous and outer layer as well as in the inner layer. The fibers included fibers of passage (black arrows) and nerve endings (open arrow). Nerve endings are characterized by a granular structure at a low magnification which is due to the axonal expansions (varicosities) close to the nerve terminal (see below). The fibers of passage did not necessarily belong to the innervation of the fascia; theoretically they may supply tissues other than the fascia. Figure 2.4.1(D,a) is a quantitative evaluation of the PGP 9.5-ir fibers. In a 5 mm long part of the fascia in coronal sections, the length of all fibers and nerve endings in the various layers of the fascia was measured and the mean fiber length was calculated. The various parts of each bar graph show the mean fiber length in a given layer. The evaluation showed clearly that the great majority of all fibers were situated in the subcutaneous tissue and outer layer of the fascia. In the middle and inner layer only a small fraction of all fibers was found. In the bar graphs subcutaneous tissue and outer layer are pooled, because the outer layer of the fascia proper was often continuous with the subcutaneous tissue. The peptidergic sensory nerve endings were identified with antibodies to the neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP; Danielson et al. 2006). CGRP is present in a high percentage of sensory fibers and serves as a general marker for these fibers (Danielson et al. 2006). The SP-containing fibers are a subpopulation of the CGRP fibers, as all SP-positive fibers also contain CGRP. Both fiber types are involved in pain processes in that they induce neurogenic inflammation, i.e., vasodilation and increase in the permeability of blood vessels caused by action potentials that release CGRP and SP from the free nerve endings of sensory fibers (Mense et al. 1996). These action potentials originate in the dorsal roots or peripheral nerve and invade the nerve endings antidromically (against the normal direction of propagation). One aim of the present study was to perform a quantitative evaluation of CGRP-ir and SP-ir nerve fibers and sensory endings. Such an evaluation is important because the density of neuropeptide-ir fibers (such as SP and CGRP) had been shown to vary greatly from one tissue to the other (McMahon et al. 1984, 1989; Brismée et al. 2009). Figure 2.4.1(D,b and c) shows a quantitative evaluation of CGRP-ir and SP-ir nerve fibers. Compared to all (PGP 9.5-ir) fibers, the CGRP fibers were much less numerous: approximately 16%. As in other tissues, the SP-ir fibers were just a small fraction of the CGRP fibers. Figure 2.4.1(B, C) shows examples of free nerve endings that were ir for these neuropeptides. A typical feature of these sensory nerve endings was the widenings of the axon (the so-called varicosities; Fig. 2.4.1B) that look like a string of beads. The varicosities are the sites where stimuli are thought to act. In the context of this article the SP-ir nerve endings are particularly interesting. As stated earlier, SP-ir fibers are assumed to be nociceptive, therefore the ending shown in Fig. 2.4.1(C) probably represented a nociceptor. In this case, it was situated in the subcutaneous tissue overlying the fascia proper. An interesting finding was that in the middle layer of the fascia no SP-ir fibers or endings were found (Fig. 2.4.1Dc). Teleologically this makes sense, because the middle layer has to transmit the forces that accompany all body movements. If nociceptive endings existed between the collagen fiber bundles of the middle layer they would be excited or even damaged (Sanchis-Alfonso & Rosello-Sastre 2000) by trunk movements. The result would be movement-induced pain even in subjects with otherwise intact low back. The paucity of fibers in the middle layer of the fascia is in line with the statements of Hagert and colleagues (2007), who distinguished between ligaments that are mechanically important yet poorly innervated (e.g., the ligaments of the wrist), and ligaments whose main role appears to be sensory. The latter structures have more loose connective tissue, and the nerve fibers are predominantly located in this tissue. Regarding the extent of sensory innervation of the TLF, the following coarse calculation can be made: The peptidergic CGRP- and SP-ir fibers are not the only sensory fibers, because there are also nonpeptidergic/lectin positive thin sensory fibers. Even if the same number of nonpeptidergic sensory fibers are added to the peptidergic ones – which is probably too high a number, because both groups overlap (Hwang & Valtschanoff, 2005) – all sensory fibers would make up approximately 1/3 of all fibers. This means that about two-thirds of the innervation of the fascia is efferent, probably consisting of sympathetic postganglionic fibers. Indeed, preliminary results from our laboratory indicated that with a specific marker for sympathetic fibers (tyrosine hydroxylase, an enzyme necessary for the synthesis of (nor) epinephrine) a large fraction of the fibers in the TLF can be labeled. This finding may explain the great influence psychological stressors have on the pain of patients with non-specific low back pain (Brage et al. 2007).
The thoracolumbar fascia as a sensory organ
Introduction
Innervation of the thoracolumbar fascia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine