CHAPTER 79 Neurotrophic Arthritis
INTRODUCTION
As noted in prior editions of this book, little has occurred in recent years to change the fact that neurotrophic arthritis of the elbow is distinctly unusual, and poorly understood and treated. Although a large number of causes of neurotrophic arthritis are recognized (Box 79-1), diagnosis is made simpler by the fact that only five or six of these causes affect the upper extremity joints, and only three occur with any frequency at all. In fact, a Medline literature search has revealed that only two case reports have appeared in the literature since 1996.48,69,73 In this section, the causes and pathogenesis of neurogenic arthropathy of the elbow are discussed, along with laboratory investigations and differential diagnosis. Arthropathy of lower limb joints and the spine will not be considered, but references cited in Box 79-1 are provided to direct the interested reader to other sources of information. A recent excellent review of the subject is available.56
PATHOGENESIS
Debate about the pathogenesis of neurotrophic arthritis began with Charcot’s paper in 1868,14 and today our understanding of the pathophysiology remains incomplete. Charcot, observing the posterior column demyelination of tabes dorsalis, suggested the loss of a trophic function protecting joints as a pathophysiologic mechanism. This view was soon challenged by those who believed that the disorder resulted from trauma. A major advance in our understanding followed the often-quoted work of Eloesser,26 who sectioned the posterior roots of the hindlimb of cats. Deafferentation combined with thermocautery of the joint cartilage led to the rapid development of neurogenic arthropathy, bone fractures, and dislocations, emphasizing the role of insensitivity and trauma. The results of chemical analysis and tests of bone strength and elasticity were normal–evidence against Charcot’s view that the joints and bones wasted from an abnormality of trophic nerves. The roles of insensitivity, activity, and trauma were further emphasized by Corbin and Hinsey,19 who found that cats with loss of sensation developed a Charcot joint only if they were allowed to roam freely in the cage, whereas those kept in a restricted space did not. These researchers were impressed by the importance of proprioceptive loss, which allowed an abnormal range of joint movement. It is also recognized that limbs rendered immobile by spasticity rarely develop an arthropathy. An important observation of Harris and Brand36 was that joint breakdown in the neuropathy of leprosy can be minimized or prevented by proper protection. Johnson37 emphasized the importance of unrecognized and untreated fractures, especially stress fractures, in the development of neurogenic arthropathy.
LOSS OF NOCICEPTION
The most widely held view on the pathogenesis of Charcot joints, then, involves a loss of protective joint nociception and position sense that subjects the joint to repeated trauma that is not recognized by the affected individual. The neurologically normal patient with an injured joint presents quickly with development of pain and loss of function, leading to immobilization and the search for appropriate medical treatment. In the neurologically impaired person, on the other hand, unrecognized injury is untreated and leads to a vicious circle of compounding injuries. This explanation is intuitively reasonable, particularly in the presence of severe superficial and deep loss of pain perception. It is more difficult to invoke when joint destruction evolves, but sensory loss is minimal or difficult to demonstrate. For example, the author has studied patients with subclinical inherited neuropathy with severe neurotrophic arthropathy of various joints and recurring fractures who had no sensory symptoms or findings on clinical examination.24 Even with sophisticated tests, including computer-assisted sensory examination, periosteal nociception, and morphometric and graded teased fiber evaluation of cutaneous nerves, only a mild neuropathic abnormality was found. Methods of measuring joint capsule and articular bone pain perception are not available; however, cortical bone is known to contain both unmyelinated and myelinated fibers, and presumably some of these subserve nociception.18 Joint capsules, particularly in their external layers, contain several types of formed corpuscles and naked nerve endings. Synovium has not been observed to contain nerve endings. Although pain sense is carried by both small myelinated and unmyelinated fibers, the relative importance of these two systems in normal protective joint sensation is unknown. In those puzzling patients in whom sensation appears to be normal, a new method of measuring joint pain perception or afferent nerve fiber activity is needed.
NEUROVASCULAR THEORY
First, a proportion of patients with Charcot joints are said to have no neurologic disease when joint destruction develops, making it difficult to blame joint insensitivity. Second, there are instances in which neurotrophic joints develop in bedridden patients in whom weight bearing is impossible and repeated severe joint trauma unlikely. Third, a few patients suffer joint destruction and resorption of articular structures in a matter of weeks to a few months, making pure mechanical destruction hard to accept as the sole mechanism.18 Fourth, some neurotrophic arthropathies are associated with long bone fractures that seemingly occur spontaneously or without unusual trauma. Although histologic examination is reported to show pathologically increased vascularity, it is also a fact that acutely damaged joints become hot and swollen from increased blood flow and exudation as part of the body’s normal repair process, making pathologic vascularity difficult to prove. Hindlimb sympathectomy might be expected to increase blood flow; however, in experimental animals, at least, it does not lead to joint damage or abnormalities in the chemical composition of bone.19 It is still possible, however, that local hyperemia is exaggerated in some patients, leading to rapid bony softening and resorption, and it is not possible to discard this hypothesis out of hand (Fig. 79-1).
INFECTION
Although infection may coexist but cannot be offered as a cause of this problem, tuberculosis of the elbow, on the other hand, can cause resorptive and destructive changes similar to those in a neurotrophic joint (Fig. 79-2).
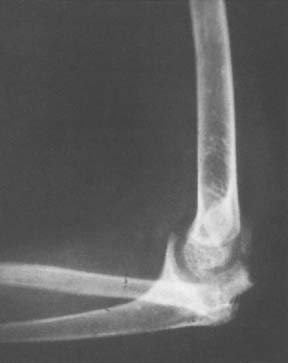
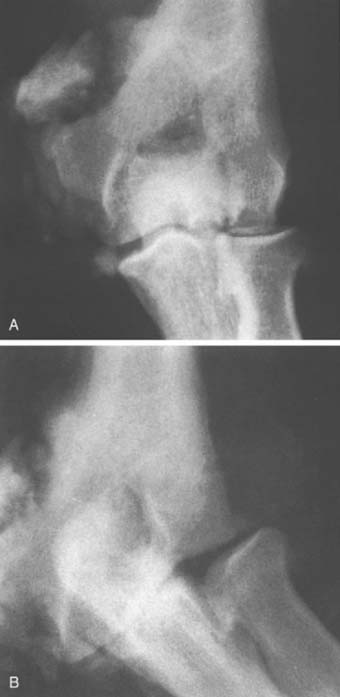
FIGURE 79-2 Gross destruction of an elbow joint involved with tuberculous arthritis. Note that both sides of the joint are involved. A distinction between this entity and a neurotrophic arthropathy in the early stages is complicated by the possibility that the two conditions coexist.47
(Courtesy of Dr. Richard Marks, Cape Town, South Africa.)
ENVIRONMENT
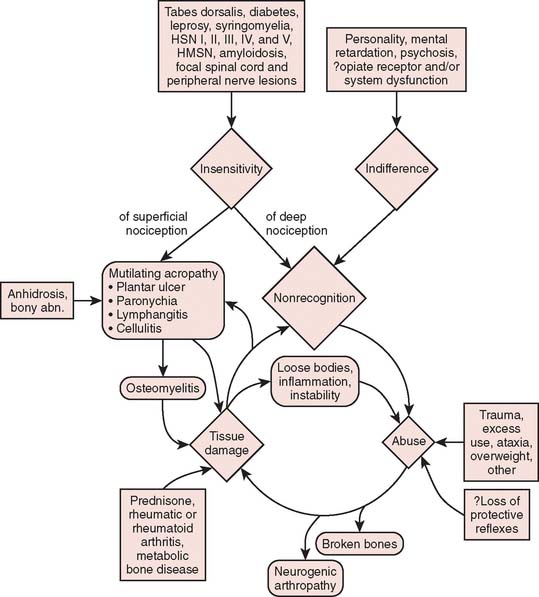
As is being more commonly recognized today, intra-articular administration of local anesthetic is associated with chondrocyte cell death.15,33 For some, it is occasionally followed by rapid joint disintegration. Patients reported with this complication have not had any neurologic disease, and therefore, the joint destruction cannot be called a neurotrophic arthropathy. The interplay of multiple factors in the genesis of bone and joint complications is emphasized in Figure 79-3.
CONDITIONS CAUSING NEUROTROPHIC ARTHROPATHY OF THE ELBOW
SYRINGOMYELIA
In contrast to tabes dorsalis, 80% of the joints involved in syringomyelia are in the upper extremities.30 It is estimated that approximately 25% of these affected joints will develop joint breakdown. Neuropathic disease involves the shoulder most commonly, followed by the elbow and wrist. Degenerative changes in the cervical spine are not uncommon. The process evolves gradually with paresthesias of the hands, followed by progressive weakness and wasting of the small hand muscles and then atrophy of arm and shoulder muscles. Loss of pain and temperature sense affects the arms and upperthoracic segments, often in a cape distribution. As the syrinx enlarges, long tract signs appear, and a Horner syndrome is noted. The Arnold-Chiari malformation is commonly associated and is related to the development of syringomyelia.
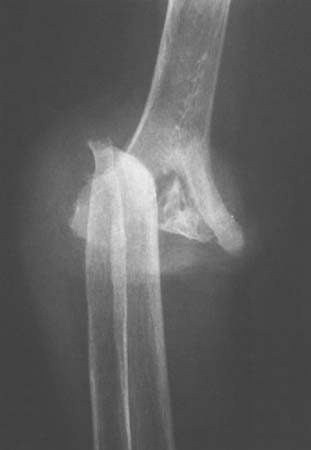
Post-traumatic syringohydromyelia presenting as a neuropathic arthropathy of the elbow and of the shoulder has been reported.51 Morrey has seen one additional instance of an acquired neurotrophic elbow joint after injury to the cervical spine (Fig. 79-4). The mechan-ism is also presumably that of a traumatic syringohydromyelia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree