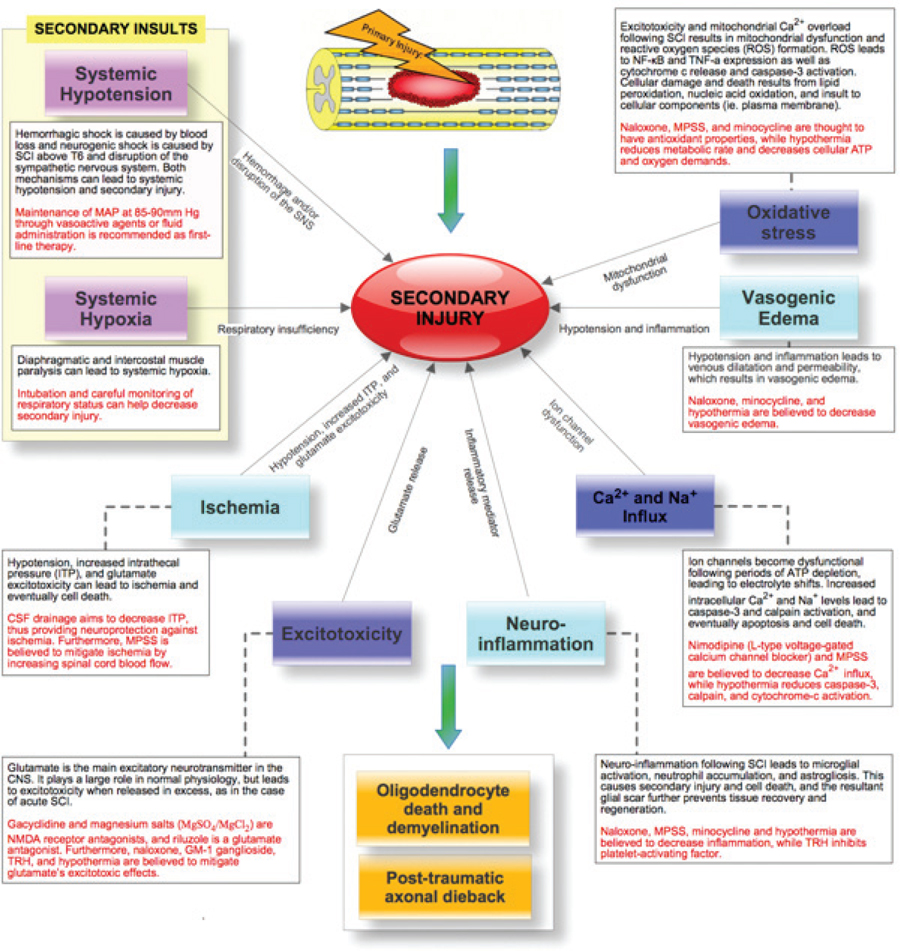
36 Key Points 1. Despite numerous drugs having been trialed for neuroprotection after SCI in the past, only MPSS has been translated to the clinic. 2. Despite not producing clinically used strategies, the trials from the past have informed research. 3. Current promising trials are being conducted on riluzole, minocycline, early surgical decompression and therapeutic hypothermia. 4. In the future, these strategies may be used in combination with neuroregenerative methods. Over the past 25 years, therapeutic strategies to protect neural tissue have emerged as a promising approach for improving outcome from spinal cord injury (SCI). Central to this approach has been the acceptance of secondary injury, which involves a period of progressive and prolonged tissue damage following the initial primary injury (Fig. 36.1). This delayed damage results from interrelated pathological cellular events, which include ischemia, excitotoxicity, electrolyte dysregulation, free radical production, mitochondrial dysfunction, and vasogenic edema leading to cell death, which are often exacerbated by systemic hypotension and hypoxia, which can accompany SCI.1–3 Following successful preclinical trials in animal models, many neuroprotective agents have been tested in human clinical trials. Recent decades have seen the trial process advance as much as the agents being tested. Thus, ongoing studies that combine promising new agents with more appropriate and sensitive outcome measurements have strong potential for human translation. Numerous pharmacological agents have been shown to have the potential to reduce secondary injury and improve functional outcomes in animal studies. However, human trials of most agents have had disappointing results, and to date, only methylprednisolone sodium succinate (MPSS) has been translated to the clinic. Nonetheless, it is important to understand current trials in the context of those that preceded them, and key trials are reviewed here. Fig. 36.1 Secondary injury mechanisms following acute spinal cord injury (SCI), and the proposed neuroprotective agents that have been studied or are planned for clinical trials in SCI to attempt to mitigate these processes. The complex secondary injury mediator interactions that follow acute SCI are only shown in part by this simplified figure. ATP, adenosine triphosphate; CNS, central nervous system; CSF, cerebrospinal fluid; ITP, intrathecal pressure; MAP, mean arterial pressure; MPSS, methylprednisolone sodium succinate; NF-kB, nuclear factor kappaB, NMDA, N-methyl-d-aspartate; ROS, reactive oxygen species; SCI, spinal cord injury; SNS, sympathetic nervous system; TNF-α, tumor necrosis factor-α; TRH, thyrotropin-releasing hormone. Gacyclidine is a noncompetitive, N- methyl-d-aspartate (NMDA) receptor antagonist that mitigates secondary injury by reducing glutamate-dependent excitotoxicity. In 1999, three escalating gacyclidine doses were tested against placebo in a phase 2 clinical trial in France.4 The study involved 280 patients with complete and incomplete SCI. A nonsignificant increase in motor function in incomplete cervical injury patients was observed. Because the overall results of the trial were negative, this line of clinical investigation was discontinued. Gacyclidine is now being investigated for the treatment of traumatic brain injury (TBI), organophosphate poisoning, and tinnitus, but it is no longer being explored for use in SCI.3 Naloxone is a competitive opioid antagonist that is believed to reduce secondary injury by antagonizing dynorphin A, a harmful endogenous opioid released following SCI.5 It also reduces edema, free-radical generation, excitotoxic amino acid release, and superoxide production by microglia.6 Following preclinical studies that suggested benefit, naloxone underwent a phase 1 human clinical trial in 1985.7 Benefit was suggested in this small trial, and in 1990, the more definitive National Acute Spinal Cord Injury Study II (NASCIS II) trial included naloxone as one of its treatment arms (alongside MPSS and placebo).8 Un-like MPSS, naloxone failed to show an improvement in motor or sensory function. Nimodipine is a dihydropyridine L-type voltage-gated calcium channel blocker that decreases intracellular calcium levels, thus inhibiting the activation of calpains and other destructive enzymes.3 A 1996 phase 3 randomized, controlled trial (RCT) involving 100 patients with complete and incomplete SCI failed to demonstrate benefit.9 Thyrotropin-releasing hormone (TRH) is a neurohormone that mitigates secondary injury by antagonizing the actions of excitotoxic amino acids, peptidoleukotrienes, endogenous opioids, and platelet-activating factor.2,10 TRH demonstrated efficacy in treating brain injury and SCI in preclinical animal studies and in 1995 was studied in a small phase 2 human SCI trial.11 Although statistically significant functional improvement was seen in incomplete (but not complete) SCI patients, patient attrition and the limited sample size necessitate cautious interpretation.3 No further trials with this agent are on the horizon despite this suggestion of benefit. GM-1 ganglioside (Sygen, Fidia Pharma USA, Inc., Parsipanny, NJ) is a member of a heterogeneous family of complex glycosphingolipids, which are abundant in neurons. GM-1 mimics endogenous neurotrophic factors, which stimulate nerve-fiber growth and repair, and it has been explored as a therapy for multiple neurodegenerative diseases.12 GM-1 may also reduce glutamate-mediated excitoxicity and subsequent apoptosis.13 A small phase 2 trial of GM-1 in SCI in 1991 suggested improved neurological recovery,14 and the following year, a larger phase 3 RCT (the Sygen Multi-Center Acute Spinal Cord Injury Study) was initiated.15 GM-1 failed to show a significant benefit, although a nonsignificant trend toward greater motility on the Modified Benzel Walking Scale was noted. A low dose of GM-1 ganglioside (300 mg loading dose and then 100 mg/d for 56 d) is recommended as an option by the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS),16 although it is not used in clinical practice due to the lack of availability of this compound. Methylprednisolone sodium succinate (MPSS) is by far the most extensively researched pharmacotherapy for SCI, having been investigated in five large, human clinical trials over the past 25 years. Despite controversy surrounding its side effects, MPSS is also the most widely used pharmacotherapy in the treatment of acute SCI. As a synthetic glucocorticoid, MPSS has antiinflammatory and antioxidant properties that mitigate oxidative stress, calcium influx, and lipid peroxidation, leading to increased oligodendrocyte survival and decreased posttraumatic axonal dieback following acute SCI.3,17 Subsequent to promising preclinical study results, a series of five human clinical trials (Table 36.1) was launched to study the efficacy, safety, and dosage of MPSS. The most influential were the three NASCIS trials, which set the foundation for current SCI treatment protocols. Despite extensive human clinical trials involving multiple centers and more than 1500 patients, expert opinions on MPSS administration remain divided. Neurological benefits were modest, inconsistent, and only apparent in post hoc analyses. Furthermore, important side effects of MPSS have been consistently seen and include pulmonary complications, gastrointestinal complications, infections, delayed wound healing, and death. Many physicians now choose not to administer MPSS,26–29 although the senior author (MGF) and many other clinicians support its use when confronted with severe neurological consequences and the lack of alternatives.3 The AANS/CNS guidelines recommend MPSS at an optional level because its modest benefits are balanced by important side effects.30 New trials combine a greater understanding of SCI with improved clinical trial methodology. Minocycline and riluzole are currently being investigated in human clinical trials, whereas magnesium salts in polyethylene glycol (PEG) showed successful preclinical results and promise for future human translation. Nonpharmacological neuroprotective strategies, including cerebrospinal fluid (CSF) drainage, early surgical decompression, and therapeutic hypothermia, are also undergoing human clinical trials. Minocycline is a synthetic tetracycline antibiotic. In addition to its bacteriostatic properties, it has antiinflammatory and antiapoptotic properties, acting to suppress cytokine production by inflammatory cells, microglial activation, and neuronal death.31,32 In animal models, it has shown efficacy in numerous neurological conditions,33 and in particular, it has decreased oligodendrocyte loss, motor axon dieback, and lesion size in SCI.34 In 2004, a phase 1/2 human clinical trial was initiated by the University of Calgary to test the efficacy of minocycline against placebo. The results of this trial have recently been published. The authors report that minocyline treatment was safe and feasible, but did not result in statistically significant improvement in patient outcomes. However, there were tendencies toward an improvement in some outcome measures and the authors conclude that a phase 3 clinical trial is warranted. Riluzole is a US Food and Drug Administration (FDA)-approved benzothiazole anticonvulsant traditionally used in the treatment of amyotrophic lateral sclerosis (ALS).35 Recently it has been applied in the treatment of other neurological conditions, including acute SCI. Riluzole antagonizes glutamate excitotoxicity by inhibiting presynaptic glutamate release and increasing high-affinity glutamate uptake, mitigating its neurotoxic mechanisms.36 Animal studies have revealed favorable results and synergy with MPSS,37 demonstrating decreased neuronal death and enhanced outgrowth of sensory neurons.38 In August 2009, a human trial investigating riluzole was initiated under the leadership of Dr. Michael Fehlings and the North American Clinical Trial Network (NACTN).22 Patients are randomized to receive riluzole (by mouth 50 mg every 12 hours) or placebo within 12 hours of injury. The study will employ the American Spinal Injury Association (ASIA) scale, the Spinal Cord Independence Measure (SCIM), and the Brief Pain Inventory in patient assessments. Magnesium sulfate and chloride recovers endogenous Mg2+ ion levels, which are presumably depleted following SCI, and leads to improved neurological functioning. Magnesium is also believed to block NMDA receptors and attenuate glutamate excitotoxicity, radical generation, and apoptosis.23 Its coadministration within PEG, a hydrophilic polymer and pharmacological excipient with independent neuroprotective properties, has led to improved neurological outcome in a rodent SCI model.39 A recent preclinical study suggests that MgSO4 and MgCl2 in PEG are equally as effective in treating acute SCI (although MgCl2 showed improved early recovery), and if administered within 4 hours, lead to a statistically significant decrease in SCI lesion size and improvement in neurological function.23 Further investigation into the efficacy of MgSO4 and MgCl2 in PEG may be achieved through human clinical trials. Surgical decompression is typically performed following SCI to relieve pressure on the damaged spinal cord and to stabilize the spine. There is debate surrounding the timing of this intervention, however. Although animal studies have strongly suggested neurological benefit from early surgical decompression, many physicians prefer to delay surgery for unstable trauma patients.40 In 1999, a multicenter retrospective study found little agreement on the optimal timing of surgical treatment and identified the need for a prospective human trial to address this issue.41 In 2003, the Surgical Treatment of Acute Spinal Cord Injury Study (STASCIS) was initiated by Drs. Michael Fehlings (University of Toronto) and Alex Vaccaro (Thomas Jefferson University). This prospective observational study analyzed neurological and functional outcomes in 313 patients receiving either early (< 24 hours postinjury) or late surgical decompression. Of the 222 patients with follow-up available at 6 months postinjury, 19.8% of patients undergoing early surgery showed a ≥ 2 grade imporvement in AIS compared to 8.8% in the late decompression group.25 Following the early promising results from the STASCIS study in support of early surgical decompression, the University of Toronto has established the practice of performing decompression by traction or open surgery immediately following SCI.3 Cerebrospinal fluid (CSF) drainage lowers intrathecal pressure (ITP) and is routinely performed in thoracoabdominal aortic aneurysm surgery to prevent spinal cord ischemia and paraplegia.42 In SCI, it is believed to increase spinal cord perfusion pressure, attenuate ischemia, and provide neuroprotection.43 A human clinical trial was recently completed to study intrathecal pressure changes before and after surgical decompression, as well as to evaluate the safety, feasibility, and efficacy of CSF drainage.43 Completed in 2009, this phase 1/2 RCT randomized 22 patients with acute SCI to receive CSF drainage versus no drainage within 72 hours of injury. CSF drainage was not associated with adverse effects, but it did not show improved neurological outcome, though this is not unexpected in a small, underpowered pilot study such as this. Therapeutic hypothermia (28°C to 32°C) has been studied for various conditions since the 1970s and has become the treatment guideline for comatose and out-of-hospital cardiac arrest patients.44 Hypothermia mitigates secondary injury by decreasing metabolic rate and the neuroinflammatory response to injury.45 It has also been shown to stabilize cell membranes and to prevent caspase-3 and cal-pain activation. In 2008, the Miami Project to Cure Paralysis began a prospective clinical trial exploring the role of systemic hypothermia (as opposed to localized cooling investigated in historic studies). Immediately following SCI, patients undergo systemic cooling with chilled intravenous saline to decrease the core body temperature to ∼ 34°C. A phase 1 pilot study, involving 14 patients with complete SCI, was completed in 2009 to assess the safety and efficacy of systemic modest hypothermia (33°C) using an intravascular cooling catheter.24 Although respiratory complications and arrhythmias were noted, systemic hypothermia showed promise as a potential neuroprotective intervention. A larger, multicenter phase 2/3 RCT involving the Neurological Emergencies Treatment Trials (NETT) group is pending approval. The larger RCT hopes to better delineate the potential efficacy as well as the risks associated with systemic hypothermia. Many putative neuroprotective treatments for acute SCI have been investigated in human clinical trials. Though only MPSS has reached the clinical realm, we have learned much from the trials completed in the past. Ongoing human clinical trials in early surgical decompression, therapeutic hypothermia, and riluzole hold great promise and may also see clinical use alone or in combination with strategies that enhance regeneration or replace lost cells.
Neuroprotective Trials in Spinal Cord Injury
 Completed Neuroprotective Trials in Spinal Cord Injury
Completed Neuroprotective Trials in Spinal Cord Injury
Pharmacological Agents
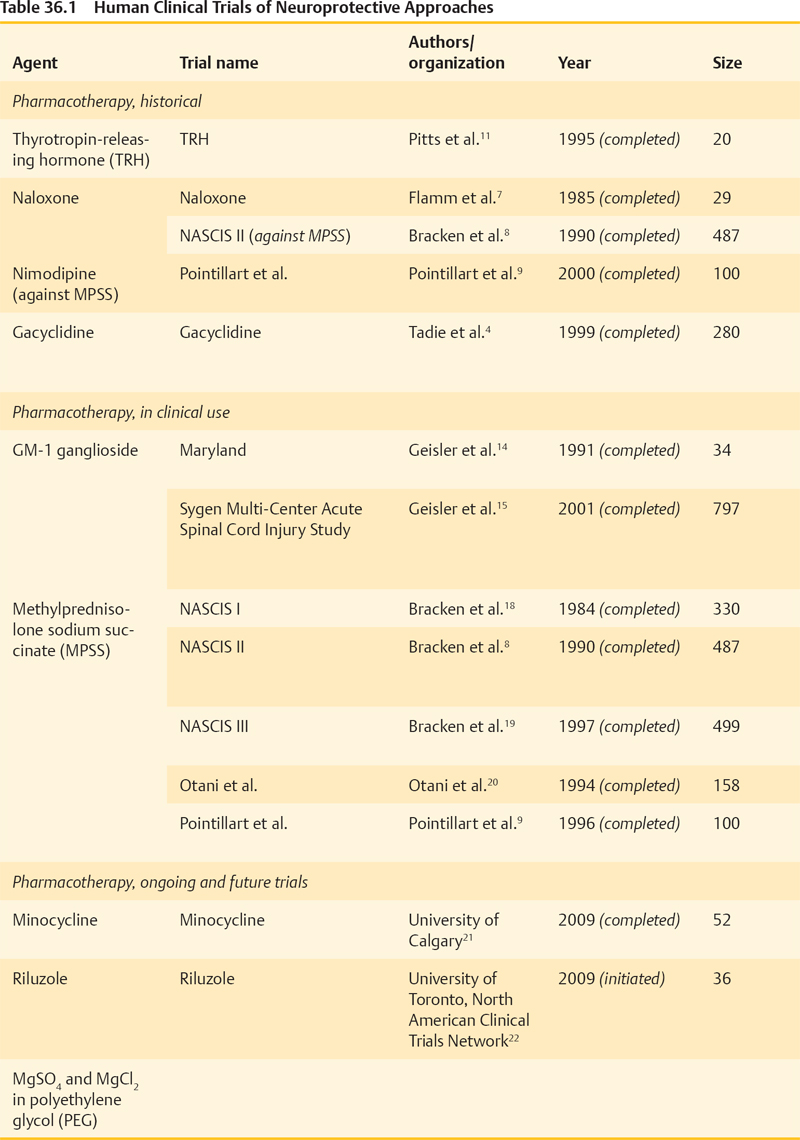
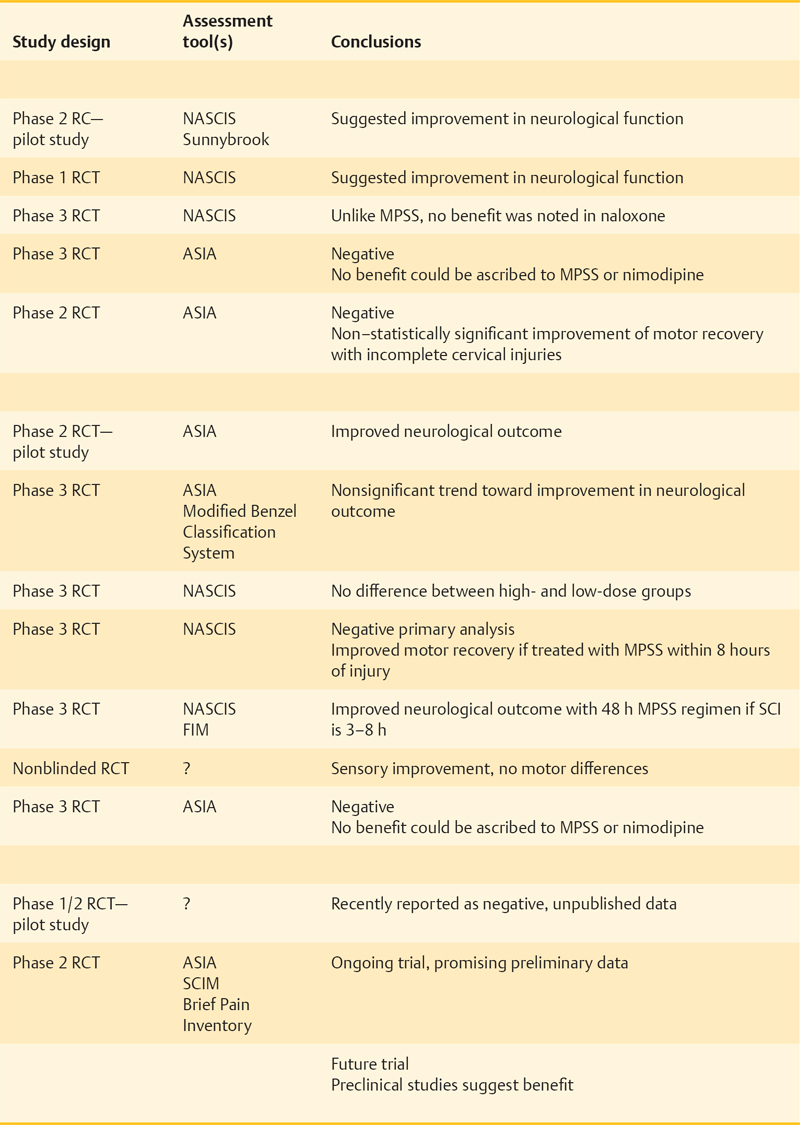

 Ongoing and Future Trials of Neuroprotective Approaches in Acute Spinal Cord Injury
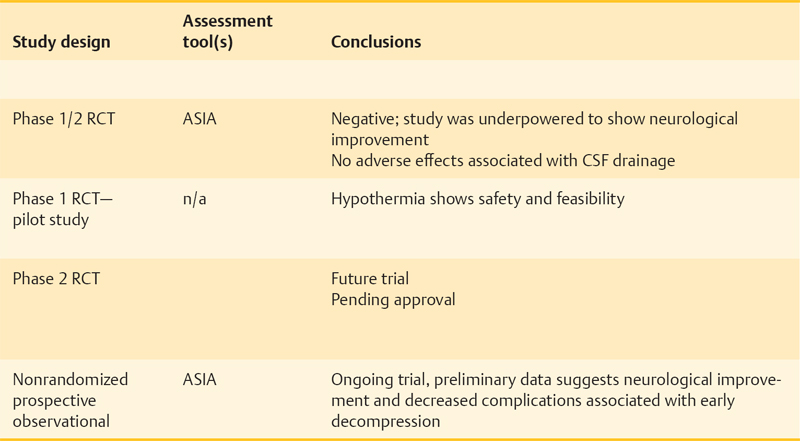
Ongoing and Future Trials of Neuroprotective Approaches in Acute Spinal Cord Injury
Pharmacological Agents
Nonpharmacological Neuroprotective Strategies
 Conclusion
Conclusion
 Subsequent to the NASCIS trials, it has been recommended that MPSS prescribed for blunt SCI less than 3 hours old should be administered as a 30 mg/kg bolus followed by a 5.4 mg/kg/h infusion for 24 hours. For injuries treated within 3 to 8 hours, a 48 hour infusion following the bolus has been recommended. It is the senior author’s (MGF) preference to use the 24 hour NASCIS II protocol to minimize the adverse side effects of MPSS on wound healing and infectious complications. MPSS is not recommended for use in SCI beyond 8 hours after injury or for penetrating SCI.
Subsequent to the NASCIS trials, it has been recommended that MPSS prescribed for blunt SCI less than 3 hours old should be administered as a 30 mg/kg bolus followed by a 5.4 mg/kg/h infusion for 24 hours. For injuries treated within 3 to 8 hours, a 48 hour infusion following the bolus has been recommended. It is the senior author’s (MGF) preference to use the 24 hour NASCIS II protocol to minimize the adverse side effects of MPSS on wound healing and infectious complications. MPSS is not recommended for use in SCI beyond 8 hours after injury or for penetrating SCI.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neuroprotective Trials in Spinal Cord Injury
