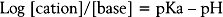CHAPTER 17 Neurophysiology of Diagnostic Injections
INTRODUCTION
In the patient presenting with symptoms in a classic dermatomal pattern and a corroborative imaging study, the diagnosis may not be in question. However, cervical radicular pain may not radiate in a classic dermatomal distribution, creating doubt in diagnosis.1 Furthermore, patients often do not present with classic symptoms. A patient presenting with posterior arm pain radiating into the radial aspect of the forearm to the wrist in the presence of multilevel cervical foraminal stenosis may have involvement of either the C6 or C7 nerve root. The ability to selectively anesthetize a specific nerve root would be helpful to determine the involved nerve root and confirm the diagnosis.
A variety of structures are potential pain generators for those patients presenting with axial pain. Potential pain generators include bone, muscle, tendon, ligament, intervertebral disc, zygapophyseal joint, and sacroiliac joint. Bone pathology can typically be diagnosed by imaging studies. Magnetic resonance imaging (MRI) has been disappointing for diagnosing discogenic pain as disc pathology can be seen in asymptomatic individuals.2–5 Furthermore, annular fissures seen on discography has been reported after normal MRI.6 Discography has been utilized to diagnose a painful disc and is discussed in greater detail in Chapter 25.
History and physical examination has not been reliable to diagnose pain of Z-joint or sacroiliac joint etiology.7–9 Imaging studies have not been helpful in diagnosing pain from the Z-joint or sacroiliac joint.10–14 Diagnosis has been based upon anesthetizing the joint.15–19 The diagnosis of mechanical low back pain has been reported to be elusive.20 However, with the advent of diagnostic injections, the etiology of mechanical low back pain can frequently be determined. With a more specific diagnosis, more specific treatment may be rendered.
PREMISE OF DIAGNOSTIC INJECTIONS
Diagnostic injections are performed to confirm or exclude a pain generator. Diagnostic injections may be utilized prior to surgery or therapeutic interventional spine management. A diagnostic injection is indicated when the diagnosis is in question despite less invasive testing and further invasive treatment is indicated.21 More specifically, when history, physical examination, imaging studies, and electrodiagnostic testing have failed to elucidate the etiology of the patient’s symptoms, a diagnostic injection may be indicated. Additionally, the patient should be a candidate for more invasive treatment such as interventional or surgical procedures. If the diagnostic injection is not going to affect treatment, the injection should not be performed.
NEUROANATOMY
The epineurium at the dorsal root ganglion consists of collagen fibrils and fibroblasts heavier than peripheral nerve as it nears transition with the thicker dura mater.22 The perineurium has multiple layers with basement membrane separating epi- and endoneurium. The endoneurium has finer collagen fibrils in the dorsal root ganglion compared to peripheral nerve.22,23
The subarachnoid angle marks the lateral border of the subarachnoid space. The dorsal root proximal to the spinal ganglion continues with epi-, peri- and endoneurium until 170 microns from the subarachnoid angle in the rat model.22 In the subarachnoid region, cells bordering the subarachnoid space may either reflect back onto itself or attach to the root sheath with punctate junctions at the subarachnoid angle. The epineurium in the subarachnoid space becomes the dura mater. The outer layers of the perineurium continue between the dura mater and arachnoid membrane. The inner layers of the perineurium becomes highly irregular. For the ventral root, highly hydrated cells, lacking basement membrane, replace the inner layers of the perineurium. In the dorsal root, the perineurium at the subarachnoid angle loses continuity with irregular tissues. In the subarachnoid space, loosely arranged cells overly the root sheath, which has endoneurial tissue similar to peripheral nerve. The basement membrane of perineurium serves as a diffusion barrier to substances such as anesthetic agents. The discontinuity of perineurium at the subarachnoid angle for the dorsal root, and lack of basement membrane for ventral root, allow easier penetration of substances to the nerve sheath.22
In the subarachnoid region, the nerve root arachnoid tissue is not as effective a barrier as perineurium to anesthetic substances. Hence, lower dosages will result in block compared to the epidural space.23 However, the subarachnoid angle may also allow quicker diffusion of anesthetics because of the discontinuity of the perineurium.
The axolemma is formed by a mosaic bilayer of primarily phospholipids with lesser amounts of glycolipids and cholesterol. The outer layers of the membrane contain the hydrophobic portion of the lipid molecule while the inner layers consist of the hydrophilic portion. Interspersed within the membrane are proteins, many of which are glycosylated. The protein moieties are fixed within the membrane and compose the ion pores or channels. Various ions such as Na+, K+, Ca+, and Cl− pass through these pores that traverse the width of the membrane. In myelinated nerve, the Na+ channels are located at the nodes of Ranvier with the K+ channels interspersed between the nodes.24 In unmyelinated nerves, the Na+ and K+ channels are not selectively located.25 The flow of ions through these channels is dependent upon various factors such as ion concentration gradient, voltage gradient, and configuration of the channel. The channels may exist in an open state, closed resting state, or closed inactivated state. The channels are ion specific, primarily only allowing the passage of a specific ion. For example, voltage-gated Na+ channels allow predominately only Na+ to pass through the channel. The voltage-gated Na+ channel is typically closed at the resting membrane potential of −60 mV, but with chemical or electrical depolarization, the transmembrane potential may reach threshold of −45 mV, resulting in opening of these voltage-gated Na+ channels. With opening, extracellular Na+ flows rapidly into the axon, resulting in an action potential with subsequent depolarization of adjacent membrane. The wave of depolarization is then propagated down the axon. In a closed state, the Na+ cannot traverse this channel.
NEUROPHYSIOLOGY
Positively and negatively charged ions are present in the intracellular and extracellular neuronal environment. Intracellularly, there is an overall negative charge and extracellularly an overall positive charge. This separation of charges results in a resting potential across the membrane. The major ions responsible for the charge are Na+, Cl−, K+, and organic anions. The organic anions, such as amino acids, remain intracellular and are not permeable to the axolemma. The concentrations of Na+ and Cl− are higher extracellularly and K+ concentrations higher intracellularly. Typically, ions will diffuse from a higher concentration to a lower concentration. Electrically, positively charged ions will tend to diffuse to the more negative side. These forces interact until equilibrium develops between the electropotential and concentration gradient. This has been termed the equilibrium potential and for each ion is dependent upon concentration gradients, electropotential gradients, ion charge, and permeability. The neural membrane affects these factors.26
A Na+–K+ pump regulates the flow of these ions with three Na+ ions pumped extracellularly to every two K+ ions pumped intracellularly. The energy-dependent pump is driven by the hydrolysis of ATP. The pump accounts for maintenance of the ion gradient across the membrane and maintains the resting potential. Without the pump, Na+ accumulates intracellularly and K+ extracellularly until the electropotential gradients for both become zero. The pump maintains the potential difference at a metabolic cost – hydrolysis of ATP. The Na+–K+ pump, high permeability of K+, and low permeability of Na+ results in a zero net influx:efflux of ions, maintaining the resting potential.26
Membrane phospholipids have an insulating property separating the negatively charged axoplasma from the positively charged extracellular fluid. The charge is separated and maintained with a negative charge on the inner membrane and positive charge on the outer membrane with a potential difference. The membrane phospholipids bilayer serves as a capacitor.27
Another factor that will affect electrical conduction longitudinally down the axon is axon diameter. With a larger axoplasmic core, more ions are available for transmission of the current, resulting in a lower resistance. Smaller axons have higher resistance. For a given voltage potential across the membrane, a higher resistance will result in lower conductance (I=V/R). Another factor is the length constant. The length constant is the length of axon that a voltage potential can spread passively. The decay of voltage down the length of axon is exponential and related to the loss of current through the membrane and resistance to current in the axoplasmic core. The higher the membrane resistance the longer the length constant will be as there is less decay in the potential. Conversely, the lower the axoplasmic resistance the longer the length constant will be. A longer length constant allows spatial summation of impulses.27 The length constant is important in allowing the propagation of a depolarizing current to adjacent sections of axon without decay.
The velocity of depolarization is dependent upon membrane capacitance and axon resistance. A lower resistance allows a larger conductance. A larger capacitance will require a larger ion flow to alter the charge to change the transmembrane potential. The velocity of depolarization down an axon is dependent upon the product of axon resistance and membrane capacitance. With increasing axon diameter, the resistance is exponentially decreased with only linear increase in capacitance. This leads to higher velocity. Another way to affect velocity favorably is to increase the thickness of the capacitor, which results in decreased capacitance. Myelination achieves this goal. Myelin will also decrease the amount of ion flow across the membrane with less decay. However, the depolarization would dissipate without the node of Ranvier to allow Na+ channel conductance. This results in saltatory conduction as the action potential jumps from node to node. Sodium channels are located at the nodes. Potassium channels are primarily located along the axon between the nodes of Ranvier with few if any potassium channels at the nodes.24 The axonal membrane between nodes functions as a passive cable unless demyelination occurs. With demyelination, the bare axon becomes excitable.25 However, demyelination can result in conduction block due to decay of the propagating action potential.
Subthreshold changes in the transmembrane potential can result in intermediate opening of only a few voltage-sensitive Na+ channels which flip between open and closed states. Additionally, these subthreshold spikes may increase K+ conductance extracellularly. This can lead to some resistance to depolarization creating a higher threshold, termed accommodation.28 Once a threshold stimulus occurs, then depolarization occurs with opening of all sodium channels with development of an action potential. The action potential is then propagated down the axon. This process allows transmission along long neural pathways in the body.
Neurophysiologic effects of local anesthetics
Local anesthetics block nerve impulses by inhibiting depolarization. Local anesthetic exists in both a neutral and cation form. The cation moiety has been determined to be the active form.29–31 The cation form binds to a receptor located on the alpha subunit of the ion-conducting pore. The receptor consists of an amino acid chain within the pore.32
The cation-receptor complex alters the configuration of the sodium channel. The influx of sodium is blocked. Depolarization does not occur and the propagating impulse is blocked. The blocked segment of nerve maintains the resting potential with resultant membrane stabilization.33 Local anesthetic then dissociates from the receptor, allowing sodium conductance to resume. The cation binds and dissociates from the receptor through open channels.34 Complexed channels open and close normally but do not conduct sodium.34 Depolarizing impulses open the channels. In the presence of local anesthetic, further binding occurs leading to greater inhibition.34 Increased inhibition with depolarization has been termed phasic inhibition. With increasing discharges, more channels are opened and subject to greater blockade.35,36 The amplitude of the conditioning impulse – larger impulse with more channels open – will affect the number of channels opened and subsequent inhibition.35 Recovery from this frequency-dependent block is dependent on the concentration of anesthetic.36
Another factor that affects whether the propagating wave of the depolarizing is aborted is the length of nerve inhibited. Previously, the inhibition of depolarization at three consecutive nodes was considered the critical length for conduction block.37,38 Local anesthetic was found to result in graded reduction in nodal action potential current. Graded reduction of the sequential nodes occurred until propagation ceased.39 Complete conduction block at three consecutive nodes was found not to be necessary. However, the concept of graded reduction across sequential nodes and complete block at three consecutive nodes are not mutually exclusive.38 The graded response is dose dependent. With higher dosages complete block of sequential nodes can occur.
Various factors affect the rapidity, density, and duration of neural blockade. The onset of blockade is affected by anesthetic permeability. The anesthetic agent needs to penetrate epineurium, perineurium, endoneurium, and axolemma. In myelinated nerves, penetration would occur through the myelin sheath or at the nodes of Ranvier. Diffusion across these structures is dependent upon the ion state of the local anesthetic. The neutral form of local anesthetic is more lipophilic and can diffuse across the phospholipid bilayer of the axonal membrane.31 Alkaline pH favors the neutral form and more readily penetrates mammalian A, B, and C fiber than the cation form.30 However, the neutral form is not the active form. The neutral form needs to be converted to the cation form for blockade to occur. Conversely, the active charged cation has greater difficulty penetrating and diffusing across the axonal membrane.29,30,40
The pKa is the pH at which an anesthetic is present equally in neutral and cation form. Intracellularly, the pH is 7.4 and will favor anesthetics with a pKa lower than this – a greater concentration of the active cation form. The onset of anesthesia is faster for agents with a lower pKa.41,42 Other factors affect the ability of local anesthetics to diffuse across nerve membrane. Besides lipid solubility, permeability is additionally affected by molecular volumes, specific chemical groups, and position of chemical groups on the molecule.42
Once the neutral form of anesthetic diffuses into the phospholipid bilayer, the anesthetic agent has to desorb from the membrane into the axolemma. The benzene ring of the local anesthetic may be strongly associated to the membrane.42 The NH-C4H9 group of tetracaine is more hydrophobic than the NH2 group of procaine, with resultant slower desorption.42 The desorption of the neutral forms is inverse to the partition coefficients. The desorption rate may be the rate-limiting factor to the onset of blockade.42
Other factors may affect the degree and duration of blockade. Anesthetics with a longer alkoxyl chain have greater hydrophobia which enhances voltage-dependent block.35 The potency between anesthetic compounds is probably secondary to different kinetics within the sodium channels during the step-depolarizing pulses.35
The differences in lipid solubility among anesthetic agents relative to impulse frequency conduction block has been evaluated in frogs.36 Low and high lipid-soluble anesthetics required large number of impulses to reach maximum block in vitro.36 Intermediate lipid-soluble agents required 4–8 impulses at 40 Hz to reach maximum effect. The recovery from blockade was quicker with the intermediate lipid-soluble agents.36 Low lipid-soluble agents were the quartenary compounds. Intermediate lipid-soluble agents were procaine, lidocaine, prilocaine, and mepivacaine. High lipid-soluble agents were bupivacaine, tetracaine, etiodocaine.36
Not all nerves have the same degree of susceptibility to anesthetic agents. Differential block refers to preferential blocking of small fibers over large fibers. The smaller fibers are blocked more easily as there is less tissue for local anesthetic to diffuse across. The unmyelinated C-fibers and smaller-diameter myelinated A-delta fiber are more easily blocked than A-alpha and beta fibers. Hence, pain is preferential blocked over light touch, pressure, and motor. A higher concentration of anesthetic is needed to block these larger fibers. However, fiber size is not the only factor that leads to differential block. Another factor that may be related to differential block is frequency-dependent block. An extremely phasic sensory nerve with short, widely spaced bursts may be resistant to block.36 Nerve firing at 10–50 Hz with burst durations greater than 0.5 seconds are more apt to be blocked.36 In a painful spine condition, the C-fibers and A-delta fibers are actively firing and may be preferentially blocked.
The minimum concentration of anesthetic agent to block a nerve in vitro is the Cm. The Cm between spinal nerve and peripheral nerve is the same.41 However, the Cm is lower for subarachnoid versus epidural blockade. Various factors account for this difference. The nerve roots within the thecal sac have less tissue and barrier to anesthetic agent. Lymphatics and the venous plexus in the epidural space can carry local anesthetic away from the site. Local tissue within the epidural space can bind local anesthetic, rendering it unavailable to the spinal nerve and nerve root. Fluid within the space can dilute anesthetic agent. Any fibrous tissue between the anesthetic agent and targeted nerve serves as a barrier that anesthetic agent has to cross. Another factor that can affect the onset of blockade is the presence of a rapid transport route between the epidural space and endoneurial space.43 This rapid transport is postulated to occur through epidural venous system with retrograde flow into intraneural capillaries of the nerve roots – bypassing diffusion across the dura. Local anesthetic in the epidural space may have direct transport to the axons of the nerve roots.43 In the performance of spinal injections, the goal is to place the anesthetic agent as close as possible to the dorsal root ganglion to help minimize these other factors. Posterior epidural injections compared to selective nerve root injections would be more prone to venous absorption, lymphatic uptake, and tissue binding. Other factors that effect Cm are the pKa, metabolism, elimination, and distribution of the anesthetic.
The molecular structure of the local anesthetic agents affects their properties. For example, exchanging the butyl group of mepivacaine for methane on the amine branch increases the protein binding affinity. This substitution creates bupivacaine which has a longer duration of neural blockade than mepivacaine secondary to improved binding to the protein receptor in the sodium channel. Changes on the aromatic head affect lipid solubility. As lipid solubility is a primary determinant of onset latency, any alteration of the aromatic head will affect the onset of neural blockade.44
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree