CHAPTER 13 Steroids in Spine Interventions
Corticosteroids currently constitute the cornerstone of spinal injections for management of painful inflammatory conditions unresponsive to oral treatment. Steroids are potent antiinflammatory medications. Originally introduced for treatment of adrenal insufficiency, they have evolved into an integral treatment of inflammatory conditions such as rheumatoid arthritis since their introduction in 1949.1 Robechhi and Capra,2 in 1952, in Italy followed by Lievre et al.3 in France provided the initial reports of the use of glucocorticoids as a therapeutic component in an epidural injection. Goebert and Gardner,4,5 in 1961 reported the first use of epidural steroid injections in the USA. Since 1961, the use of locally acting injectable steroids in the treatment of painful spine conditions has expanded steadily. Steroids are currently used in a variety of spine injection procedures. They are used in caudal, interlaminar, and transforaminal epidural injections, intrathecal injections,6 intra-articular facet joint injections, and in intra-articular sacroiliac joint injections. Although other therapeutic agents are used for spine injections, the frequency of steroid use surpasses the use of all other agents by far.
STEROID PHYSIOLOGY
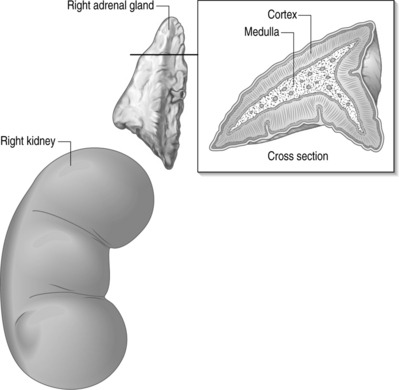
The adrenal gland is comprised of two distinct anatomical and histologic parts, the cortex being on the periphery while the medulla is located centrally (Fig. 13.1). The cortex is considered a component of the endocrine system normally producing steroids, hence their name corticosteroids. The medulla is considered a modified ganglion and a component of the sympathetic nervous system, producing epinephrine, norepinephrine, and dopamine.
The zona fasciculata predominantly produces the glucocorticosteroids, cortisol and corticosterone, due to an abundance of 3β-hydroxysteroid dehydrogenase enzyme activity. The main endogenous glucocorticosteroid produced is cortisol. It is produced at a daily rate of 10 mg/day.7 The production of the cortisol is regulated by the hypothalamus and the pituitary gland using a negative feedback mechanism. The hypothalamus produces corticotropin releasing hormone (CRH) which releases adrenocorticotrophic (ACTH) hormone from the anterior pituitary gland. ACTH in turn stimulates the zona fasciculata to produce cortisol.
Ninety percent of cortisol is reversibly bound in the circulation to an α-globulin called transcortin or corticosteroid-binding globulin (CBG) with a small amount of binding to albumin. CBG has a high affinity to cortisol leaving corticosterone relatively less bound. The half-life of cortisol in the circulation is about 1 hour. Cortisol is metabolized mostly in the liver and to a lesser extent in the kidneys. Steroid metabolism involves sequential additions of oxygen and hydrogen atoms followed by conjugation with sulfates or glucoronides to form water-soluble and inactive products. These products are predominantly excreted in urine.
ACTION OF STEROIDS
Antiinflammatory effects
The antiinflammatory action of epidural steroids is suggested to be secondary to:8
Synthetic steroids are more potent than endogenous cortisol and possess variable glucocorticoid and mineralocorticoid activity. Chemically engineered modifications have generated a variety of steroid compounds with different glucocorticoid and mineralocorticoid activity, enhanced potency, and longer duration of action. Since glucocorticoids act at the same receptors, it is not yet possible to separate the antiinflammatory effects from the action on carbohydrate, protein, and fat metabolism, much less its effect on the hypothalamus–pituitary axis. To achieve an effective therapeutic level of serum glucocorticoid, the administration of high oral or parenteral doses may be necessary. The administration of such doses produces the deleterious systemic side effects and simultaneously inhibits ACTH secretion that can lead to adrenal insufficiency.
SYSTEMIC ABSORPTION
It has been proposed and widely accepted that the selective administration of steroids, such as in epidural, perineural, or intra-articular injections produces high local concentration of the steroids without significant systemic absorption. The consensus is that systemic absorption after spine procedures is insufficient to cause serious systemic side effects. We observe through our clinical experience that the most commonly reported systemic side effect of steroids after spine injections is temporary hyperglycemia in diabetics. A few patients experience temporary hypertension, insomnia, nervousness, and rash in the anterior chest and face. The issue of systemic absorption of epidural injections and the systemic side effects are the subject of many studies. There are several reports suggesting that following epidural steroid injections hypothalamic–pituitary–adrenal axis suppression occurs for a period of 4 days to 6 weeks.9,10 Tuel et al. also reported the development of cushingoid syndrome after the epidural use of methylprednisolone.64 Janicki et al.12 analyzed the pharmacokinetics of methylprednisolone after epidural administration in rabbits of different doses of 1.25, 2.5 and 5 mg/kg. At 6 and 12 hours after administration, plasma levels of methylprednisolone was detectable only after administration of the highest dose (5mg/kg) and was undetectable at 24 and 72 hours. Plasma methylprednisolone levels were not detectable for doses 1.25 mg and 2.5 mg/kg at all sampling times. Jacobs et al.13 evaluated systemic steroid absorption after a single epidural steroid injection of methylprednisolone acetate 80 mg, and found no significant systemic absorption. While the extreme systemic complications that can ensue following the local deposition of glucocorticoids in the spinal canal are rare, this does not in any way suggest that the effects are all local. Indeed, a recent study, by Bhat et al.14 unequivocally demonstrated that transforaminal epidural steroid instillation results in a transient insignificant, but nevertheless, systemic side effect of dysphonia or hoarseness.
Ward et al.15 studied systemic side effects following the use of epidural steroids. Ten patients with sciatica underwent a short insulin tolerance test, when fasting glucose, insulin, and cortisol concentrations were measured before and twice following (at 24 h and 1 week) a caudal epidural of 80 mg triamcinolone. Serum glucose after insulin administration declined from 3.6% μ min before the epidural to 1.9% μ min 24 h afterward and returned to pretreatment values by 1 week. Significantly elevated fasting insulin and glucose levels also reflected impaired insulin sensitivity immediately after the epidural injection. Furthermore, morning cortisol levels were suppressed after the epidural injection (49 nmolul at 24 h and 95 nmolul at 1 week vs. 352 nmolul at baseline).
Hsu et al.16 evaluated the plasma cortisol and ACTH profiles after a single epidural injection of 40 mg and 80 mg of triamcinolone. Plasma cortisol was markedly reduced for only 24 hours, whereas the group receiving 80 mg of triamcinolone showed reduction for up to 14 days, with hypothalmic–pituitary axis (HPA) returning to normal at 35 days in both groups.
STEROID COMPLICATIONS AND SIDE EFFECTS
Local side effects after spinal injections
Steroid side effects occurring after spine injections have been reported; however, they are considered to be clinically uncommon compared to the number of procedures performed. The reported side effects are either due to the steroid itself or components of the steroid preparation. A number of complications are due to technical errors in performing the procedures, and this is discussed in detail elsewhere in this book. One report suggested that steroid injections can have a detrimental effect on the function of the inflammatory process, which in its turn suppresses the resorption of disc herniations.16a Intradiscal injection of methylprednisolone acetate and its vehicle polyethylene glycol in rabbits caused degeneration and primary calcification of the discs.17 Polyethylene glycol and other glycols present in steroid preparations have been reported to cause degenerative changes in the nervous tissues and even arachnoiditis when injected into the subarachnoid space.18–21 In contrast, many reports dispute this notion.6,22,24 Abram and O’Connor reviewed 65 studies of epidural and subarachnoid injections from 1960 to 1994; they reported no incidence of adhesive arachnoiditis in patients after receiving epidural steroid injections.24 There are studies that demonstrate a beneficial effect of injected steroids in the treatment of arachnoiditis.6,23
Although anaphylactic reactions were reported in patients using parentral corticosteroids, allergic reactions rarely occur after spinal steroid injections. Allergic reactions may be secondary to the paraben preservative present in some injectable preparations. Some injectables such as betamethasone and hydrocortisone contain sulfites, which may cause allergic reactions including anaphylaxis and asthma.25
Facial flushing and generalized erythema, a warm sensation or fever less than 100°F after epidural and intra-articular steroid injections have also been reported26,27,28 with an incidence between 1.4%26 and 9.3%.29 Antihistamines, when used, improve facial flushing.26 Facial flushing after epidural steroid injections is reported to last from 12 hours29 to 72 hours.26 Insomnia, nonpositional headache, and temporary increase in pain were also reported.28
Spinal cord injury has been reported after lumbosacral nerve root block with steroid injections.30 This is postulated to be secondary to an injury or an injection of particulate steroids involving an aberrant artery of Adamkiewicz. Spinal epidural lipomatosis is another rare spinal complication reported after multiple epidural steroid injections. Roy-Camille31 reported the development of epidural lipomatosis in a patient after 103 epidural steroid injections and in another after oral steroid intake.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree