Death rates related to cancer have steadily decreased over the past few decades, and as a result, the number of survivors has exponentially increased. Increasingly, more and more secondary complications caused by cancer and its treatments are being recognized. Neuromuscular complications related to the underlying cancer itself, or caused by associated treatments, such as chemotherapy and radiation therapy, are common but are likely underreported. While neurologic involvement can occur in both the central and peripheral nervous systems at any level, this article focuses on the effects of cancer on the peripheral nervous system.
Despite numerous advances in detection and treatment, cancer remains a major health problem in the United States. One in four deaths in the United States is caused by cancer, and cancer is the leading cause of death in individuals under 85 years of age . However, death rates related to cancer have steadily decreased over the past few decades, and as a result, the number of survivors has exponentially increased. Increasingly, more and more secondary complications caused by cancer and its treatments are being recognized. Neuromuscular complications related to the underlying cancer itself, or caused by associated treatments, such as chemotherapy and radiation therapy, are common but are likely underreported. While neurologic involvement can occur in both the central and peripheral nervous systems at any level, this article focuses on the effects of cancer on the peripheral nervous system.
Using electrodiagnostic studies, neuromuscular abnormalities have been clinically detected in 2.5% to 5.5% of patients with lung or breast cancer, and in 28.5% of patients with various neoplasms . Classification of these abnormalities can be organized either by etiology or by anatomic level. Many etiologies are possible. These include direct compression or infiltration, hematogenous spread, lymphatic spread, meningeal dissemination, or perineural spread. Peripheral nervous system involvement can also be caused by paraneoplastic syndromes, or from common secondary effects related to cancer, such as malnutrition, weight loss, or infection. Acquired neuropathies can result from side effects of the cancer treatments themselves, including surgery, chemotherapy, radiation therapy, hematopoietic stem cell transplantation, or immunologic therapy. Finally, patients may have pre-existing neurologic conditions, such as diabetic polyneuropathy, that can be exacerbated by cancer or its related treatments. Often, a combination of etiologies can be recognized in individual patients.
Involvement can occur at any level of the peripheral nervous system, including the anterior horn cells, nerve roots, sensory ganglia, brachial or lumbosacral plexus, single or multiple peripheral nerves, neuromuscular junction, and the muscle. Often multiple levels are involved. Neural damage at the cellular level may take place at the cell body, axon, myelin, or a combination of all of the above. The expected clinical findings are dependent on the location of the lesion.
Direct neuromuscular effects of cancer
Radiculopathy
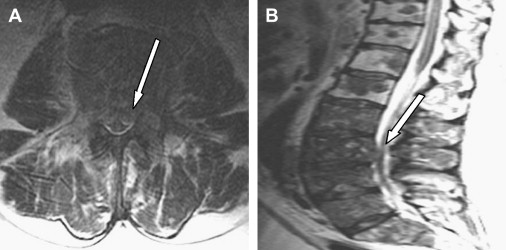
A single or multilevel radiculopathy can result from primary or epidural metastatic tumor extension into the neural foramina ( Fig. 1 ). All tumor types can metastasize to the spine, although the most common primary malignancies that do so include breast, lung, prostate, colon, thyroid, and kidney. Common primary malignant spinal tumors include multiple myeloma, plasmacytoma, and Ewing’s and osteogenic sarcoma. After disc disease and spinal stenosis, tumors involving the spine and spinal cord are the most common causes of radiculopathy . Although a degenerative etiology is more likely to cause symptoms, any patient with a history of cancer who presents with back pain and radicular symptoms and signs on physical examination warrants imaging, with magnetic resonance imaging (MRI) being the imaging modality of choice. An MRI of the total spine, not solely of the affected area, is subsequently obtained to determine extent of disease in instances where a neoplastic radiculopathy is identified.
Leptomeningeal metastases
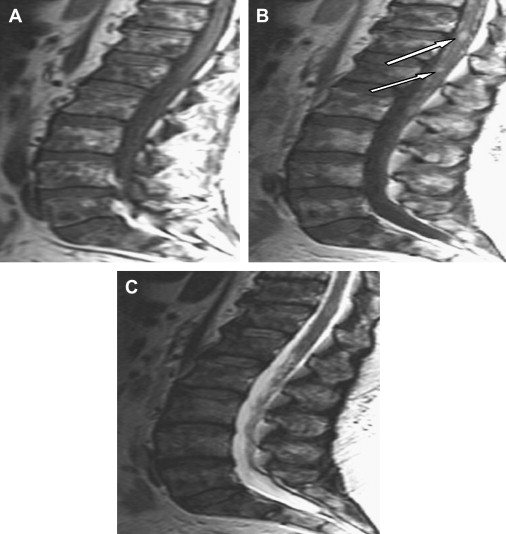
Leptomeningeal disease is caused by metastatic involvement of the leptomeninges from infiltrating cancer cells. The incidence of leptomeningeal metastasis ranges from 4% to 15% and is felt to be increasing . The most common associated primary cancers are breast, lung, gastric, melanoma, and lymphomas . Of the leukemias, leptomeningeal disease is most commonly seen in acute lymphocytic leukemia . Patients can present with an asymmetric array of symptoms resulting from polyradicular involvement, including focal and radicular pain, areflexia, paresthesias, and lower motor neuron weakness. There may be associated findings of nuchal rigidity, as well as upper motor neuron signs, especially if there is concomitant brain involvement. Cranial nerves are often involved as well, with the oculomotor, facial, and auditory nerves most commonly affected.
MRI with gadolinium of the spine and brain should be performed initially in all suspected cases. Nodular enhancement of the leptomeninges is almost pathognomonic ( Fig. 2 ). The diagnosis is confirmed with the presence of malignant cells on cerebrospinal fluid (CSF) cytology. However, there is a high initial false-negative rate on CSF studies of 40% to 50% . Repeat CSF studies following an initial negative result improves the diagnostic yield to 90% . Electrodiagnostic studies are consistent with a polyradiculopathy; however, underlying findings of an axonal, sensorimotor polyneuropathy, caused by prior chemotherapy treatment, can often be noted and can confuse the issue. Absent F-waves or prolonged F-wave latencies on nerve conduction studies are felt to be an early indicator of nerve root involvement, but are not specific for leptomeningeal disease . Treatment is palliative and involves focal radiation therapy and chemotherapy, either intrathecal or systemic. Overall prognosis is poor and is dependent on multiple factors, including primary tumor type, extent of CSF disease as well as systemic disease, degree of neurologic deficit, and associated medical comorbidities.
Plexopathy
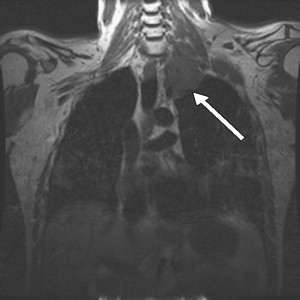
Brachial plexopathies from neoplasms are usually the result of metastatic disease, with breast and lung being the most common primary sources . In cancer patients, the frequency of neoplastic brachial plexopathy is 0.43% . If a patient has a history of prior radiation therapy to the axillary or supraclavicular lymph nodes, secondary radiation-induced neoplasms, such as sarcomas, should also be considered. Symptoms include pain, paresthesias, numbness, and weakness in the distribution of plexus involvement. Metastases can involve any portion of the brachial plexus, but usually involve the lower trunk because of its proximity to axillary lymph nodes and the superior sulcus of the lung. Assessment of T1 fibers with needle electromyography is essential and can help guide further imaging studies . The Pancoast syndrome is a distinct clinical presentation resulting from a superior pulmonary sulcus tumor, presenting with findings of a lower trunk brachial plexopathy and a unilateral Horner’s syndrome . MRI of the brachial plexus is usually diagnostic ( Fig. 3 ).
Neoplastic lumbosacral plexopathies can result from metastatic disease, but are much more likely to be caused by direct extension of local tumor or perineural spread . Common tumors involved include colon, gynecologic tumors, lymphomas, and sarcomas. As in brachial plexopathies, neuropathic symptoms will be in the distribution of involvement, and MRI of the lumbosacral plexus is helpful in the diagnosis.
Neuropathy
Mononeuropathies most often result from the direct compression or invasion from tumor, such as an isolated radial neuropathy caused by a primary osteogenic sarcoma, or a bone metastasis involving the spiral groove of the humerus. Malignant nerve sheath tumors are rare and usually arise from plexiform neurofibromas . There is a high association with neurofibromatosis type 1. The clinical presentation depends on the individual nerve involved, but severe pain and rapidly growing tumors suggest malignant transformation .
Diffuse peripheral nerve infiltration from cancer is rare but has been reported in hematologic malignancies, such as non-Hodgkin’s lymphoma and chronic lymphocytic leukemia . Amyloid deposition in primary amyloidosis and multiple myeloma can also result in diffuse polyneuropathy .
Myopathy
Focal myopathies from tumor involvement are rare, and usually result from direct infiltration from underlying bony metastases or local lymph node involvement, rather than from hematogenous spread. A more proximal myopathy, associated with macroglossia and muscle pseudohypertrophy, is an uncommon manifestation of primary amyloidosis. Muscle biopsy is diagnostic and demonstrates amyloid deposition surrounding muscle fibers and blood vessels. The selection of muscle to biopsy can be guided by electrodiagnostic findings. Needle electromyography demonstrates myopathic motor unit potentials or a mixture of large and small motor unit potentials, with fibrillation potentials noted primarily in proximal muscles .
Paraneoplastic syndromes
Neuromuscular paraneoplastic syndromes cause damage to the peripheral nervous system as a result of remote effects from a malignant neoplasm or its metastases . Although rare, it is important to recognize these syndromes. The clinical presentation is usually more rapidly progressive and severe than what would normally be expected in a noncancerous etiology. They often precede the diagnosis of cancer, and early recognition may increase survival. Treatment of the underlying malignancy usually results in improvement of neurologic symptoms. In some disorders, neuronal antigens expressed by the tumor result in an autoimmune response against both the tumor as well as healthy neural tissue, and identification of these markers can help facilitate the diagnosis of a primary tumor. Although some syndromes are associated with an identifiable neuro-oncologic auto-antibody, frequently no such marker is detected.
Almost all tumor types have been associated with paraneoplastic syndromes, and any part of the nervous system can be affected. There are, however, certain tumors that have a higher association with paraneoplastic syndromes, with neuroblastoma most often seen in children and small-cell lung cancer most often seen in adults. Paraneoplastic opsoclonus-myoclonus occurs in 2% to 3% of children with neuroblastoma. A small number (1%–3%) of patients with small-cell lung cancer develop Lambert-Eaton myasthenic syndrome (LEMS) or some other paraneoplastic syndrome .
Sensory neuronopathy
Paraneoplastic sensory neuronopathy or ganglionopathy presents with either an acute or insidious onset of pain and sensory loss. Clinical findings of sensory ataxia and pseudoathetosis are often present at various levels of severity. The findings can be diffuse but are commonly more severe in the upper extremities and may be asymmetric. Motor dysfunction is usually absent; however, sensory neuronopathy can sometimes be seen, along with a more diffuse paraneoplastic neurologic syndrome involving encephalomyelitis, autonomic neuropathy, and motor neuronopathy . A pattern of more severe sensory abnormalities on nerve conduction studies in the upper extremities, compared with the lower extremities, helps distinguish this entity from a length-dependent sensory neuropathy. The most common associated neoplasm is small-cell lung cancer; however, breast, renal, chondrosarcoma, and lymphoma have also been implicated. The presence of anti-Hu antibodies helps support the diagnosis of paraneoplastic sensory neuronopathy.
Sensorimotor polyneuropathy
The diagnosis of a true paraneoplastic distal, symmetric, sensorimotor polyneuropathy is difficult to confirm, as there are many more likely known etiologies that can cause this pattern of involvement, including diabetes mellitus, nutritional deficiencies, and toxic exposure, such as chemotherapy. A subacute, sensorimotor polyneuropathy as a paraneoplastic syndrome is therefore a diagnosis of exclusion. Symptoms include pain, paresthesias, numbness, and weakness in a stocking-glove distribution, along with hyporeflexia. A more rapidly progressive course may be the only distinguishing factor differentiating a paraneoplastic syndrome from an idiopathic or diabetic etiology. Electrodiagnostic findings are consistent with an axonal process. This syndrome has been associated with lung and breast cancer .
Vasculitic neuropathy
A pattern of clinical and electrophysiologic involvement resembling mononeuritis multiplex may represent a paraneoplastic vasculitic neuropathy. This syndrome has been most commonly reported in association with small-cell lung cancer and lymphoma . Further support for a vasculitis includes an elevated erythrocyte sedimentation rate, and an elevated cerebrospinal fluid protein level. The anti-Hu antibody has also been associated with this syndrome . Biopsy of the sural nerve confirms microvascular involvement. In addition to treating any underlying malignancy, the neuropathic symptoms may also respond to immunosuppressive therapy directed against the vasculitis.
Lambert-Eaton myasthenic syndrome
LEMS is a presynaptic disorder of neuromuscular transmission, and is perhaps the best understood paraneoplastic neuromuscular syndrome. Clinically, patients present with fatigue, proximal weakness, hyporeflexia, and autonomic dysfunction. Repetitive strength testing may reveal a “warming-up” phenomenon, where one can display an initial increase in strength with repetition followed by eventual fatigue. Bulbar involvement is rare. LEMS tends to affect adults greater than 40 years of age, and has a male predominance. It can occur independent from cancer, but up to 40% to 60% of cases have been shown to be associated with small-cell lung cancer . LEMS has also been reported to be associated with lymphoma, breast, ovarian, pancreatic, and renal malignancies.
Electrodiagnostic studies are invaluable in the diagnosis of LEMS. Motor responses are reduced in amplitude at baseline. Sensory responses are normal. Repetitive stimulation of motor nerves at low frequency (2 Hz–3 Hz) demonstrates a further decrement in amplitude. Following brief isometric exercise, facilitation occurs and compound muscle action potential amplitudes show at least a 100% increase . This finding is almost pathognomonic for LEMS (see Fig. 3 ). Antibodies directed against the P/Q-type voltage-gated calcium channels are seen in up to 92% of LEMS patients . Management involves administration of 3, 4 diaminopyridine and treatment of any underlying malignancy.
Myasthenia gravis
Myasthenia gravis (MG) is a postsynaptic disorder of neuromuscular transmission and its relationship with benign thymomas is widely recognized. MG occurs in 30% of patients with thymoma, and 15% of patients with MG are found to have thymoma on further radiographic evaluation . Patients present with fatigue and proximal weakness, most notably in ocular and bulbar muscles. Electrodiagnostic studies demonstrate a decremental response in compound muscle potential amplitude with 2-Hz to 3-Hz repetitive stimulation. Unlike LEMS, baseline motor amplitudes are normal in MG, except in severe cases. Immediately following brief exercise, a repair of the decrement is noted. Postactivation exhaustion, with return of the decremental response, is noted 2 to 4 minutes after exercise. Patients under 60 years of age with generalized weakness, or patients with a documented thymoma, are treated via thymectomy . Treatment can also involve the use of cholinesterase inhibitors or immunosuppressive agents.
Syndromes of neuromuscular hyperactivity
Hyperactivity syndromes, such as Stiff-person syndrome or neuromyotonia (Isaac’s syndrome), are rare but have been associated with malignancies, including small-cell lung cancer, breast cancer, lymphoma, and invasive thymoma . Stiff-person syndrome is a disorder characterized by muscle rigidity and a worsening of symptoms with exposure to certain triggers, such as loud noise or startle. Continuous motor unit activity is noted on needle electromyography, but otherwise electrodiagnostic findings are unremarkable. Antibodies to glutamic acid decarboxylase are present in up to 60% of patients; in some instances there is an association with antibodies against the presynaptic cell membrane protein amphiphysin. Isaac’s syndrome is an autoimmune channelopathy that has been reported in association with Hodgkin’s lymphoma as well as plasmacytoma . Antibodies to voltage-gated potassium channels are present in 50% of patients. Continuous motor unit activity is again noted on needle EMG, however unlike Stiff-person syndrome, the symptoms and findings persist during sleep. Neurotonic discharges may also be present.
Myopathy
The findings of a symmetric, proximal myopathy on clinical examination and electrodiagnostic testing can also lead to the discovery of an undiagnosed cancer. Although their classification as a true paraneoplastic syndrome is controversial, polymyositis, and especially dermatomyositis, are associated with an increased incidence of malignancy compared with the general population . Breast, lung, and gynecologic malignancies are most frequently implicated. Paraneoplastic necrotizing myopathy and carcinoid myopathy are syndromes distinct from polymyositis or dermatomyositis. Carcinoid tumors may be associated with a progressive myopathy that has its onset years after the carcinoid syndrome .
Motor neuron disease
With regard to motor neuron disease syndromes, as mentioned previously the anti-Hu-associated paraneoplastic encephalomyelitis, sensory neuronopathy, and motor neuropathy syndrome has a strong link with small-cell lung cancer. Subacute motor neuropathy and primary lateral sclerosis have been associated with lymphoma and breast cancer, respectively . There is no known association between cancer and amyotrophic lateral sclerosis; however, in newly diagnosed motor neuron disease a screening for cancer is usually part of the exclusionary diagnostic workup.
Paraneoplastic syndromes
Neuromuscular paraneoplastic syndromes cause damage to the peripheral nervous system as a result of remote effects from a malignant neoplasm or its metastases . Although rare, it is important to recognize these syndromes. The clinical presentation is usually more rapidly progressive and severe than what would normally be expected in a noncancerous etiology. They often precede the diagnosis of cancer, and early recognition may increase survival. Treatment of the underlying malignancy usually results in improvement of neurologic symptoms. In some disorders, neuronal antigens expressed by the tumor result in an autoimmune response against both the tumor as well as healthy neural tissue, and identification of these markers can help facilitate the diagnosis of a primary tumor. Although some syndromes are associated with an identifiable neuro-oncologic auto-antibody, frequently no such marker is detected.
Almost all tumor types have been associated with paraneoplastic syndromes, and any part of the nervous system can be affected. There are, however, certain tumors that have a higher association with paraneoplastic syndromes, with neuroblastoma most often seen in children and small-cell lung cancer most often seen in adults. Paraneoplastic opsoclonus-myoclonus occurs in 2% to 3% of children with neuroblastoma. A small number (1%–3%) of patients with small-cell lung cancer develop Lambert-Eaton myasthenic syndrome (LEMS) or some other paraneoplastic syndrome .
Sensory neuronopathy
Paraneoplastic sensory neuronopathy or ganglionopathy presents with either an acute or insidious onset of pain and sensory loss. Clinical findings of sensory ataxia and pseudoathetosis are often present at various levels of severity. The findings can be diffuse but are commonly more severe in the upper extremities and may be asymmetric. Motor dysfunction is usually absent; however, sensory neuronopathy can sometimes be seen, along with a more diffuse paraneoplastic neurologic syndrome involving encephalomyelitis, autonomic neuropathy, and motor neuronopathy . A pattern of more severe sensory abnormalities on nerve conduction studies in the upper extremities, compared with the lower extremities, helps distinguish this entity from a length-dependent sensory neuropathy. The most common associated neoplasm is small-cell lung cancer; however, breast, renal, chondrosarcoma, and lymphoma have also been implicated. The presence of anti-Hu antibodies helps support the diagnosis of paraneoplastic sensory neuronopathy.
Sensorimotor polyneuropathy
The diagnosis of a true paraneoplastic distal, symmetric, sensorimotor polyneuropathy is difficult to confirm, as there are many more likely known etiologies that can cause this pattern of involvement, including diabetes mellitus, nutritional deficiencies, and toxic exposure, such as chemotherapy. A subacute, sensorimotor polyneuropathy as a paraneoplastic syndrome is therefore a diagnosis of exclusion. Symptoms include pain, paresthesias, numbness, and weakness in a stocking-glove distribution, along with hyporeflexia. A more rapidly progressive course may be the only distinguishing factor differentiating a paraneoplastic syndrome from an idiopathic or diabetic etiology. Electrodiagnostic findings are consistent with an axonal process. This syndrome has been associated with lung and breast cancer .
Vasculitic neuropathy
A pattern of clinical and electrophysiologic involvement resembling mononeuritis multiplex may represent a paraneoplastic vasculitic neuropathy. This syndrome has been most commonly reported in association with small-cell lung cancer and lymphoma . Further support for a vasculitis includes an elevated erythrocyte sedimentation rate, and an elevated cerebrospinal fluid protein level. The anti-Hu antibody has also been associated with this syndrome . Biopsy of the sural nerve confirms microvascular involvement. In addition to treating any underlying malignancy, the neuropathic symptoms may also respond to immunosuppressive therapy directed against the vasculitis.
Lambert-Eaton myasthenic syndrome
LEMS is a presynaptic disorder of neuromuscular transmission, and is perhaps the best understood paraneoplastic neuromuscular syndrome. Clinically, patients present with fatigue, proximal weakness, hyporeflexia, and autonomic dysfunction. Repetitive strength testing may reveal a “warming-up” phenomenon, where one can display an initial increase in strength with repetition followed by eventual fatigue. Bulbar involvement is rare. LEMS tends to affect adults greater than 40 years of age, and has a male predominance. It can occur independent from cancer, but up to 40% to 60% of cases have been shown to be associated with small-cell lung cancer . LEMS has also been reported to be associated with lymphoma, breast, ovarian, pancreatic, and renal malignancies.
Electrodiagnostic studies are invaluable in the diagnosis of LEMS. Motor responses are reduced in amplitude at baseline. Sensory responses are normal. Repetitive stimulation of motor nerves at low frequency (2 Hz–3 Hz) demonstrates a further decrement in amplitude. Following brief isometric exercise, facilitation occurs and compound muscle action potential amplitudes show at least a 100% increase . This finding is almost pathognomonic for LEMS (see Fig. 3 ). Antibodies directed against the P/Q-type voltage-gated calcium channels are seen in up to 92% of LEMS patients . Management involves administration of 3, 4 diaminopyridine and treatment of any underlying malignancy.
Myasthenia gravis
Myasthenia gravis (MG) is a postsynaptic disorder of neuromuscular transmission and its relationship with benign thymomas is widely recognized. MG occurs in 30% of patients with thymoma, and 15% of patients with MG are found to have thymoma on further radiographic evaluation . Patients present with fatigue and proximal weakness, most notably in ocular and bulbar muscles. Electrodiagnostic studies demonstrate a decremental response in compound muscle potential amplitude with 2-Hz to 3-Hz repetitive stimulation. Unlike LEMS, baseline motor amplitudes are normal in MG, except in severe cases. Immediately following brief exercise, a repair of the decrement is noted. Postactivation exhaustion, with return of the decremental response, is noted 2 to 4 minutes after exercise. Patients under 60 years of age with generalized weakness, or patients with a documented thymoma, are treated via thymectomy . Treatment can also involve the use of cholinesterase inhibitors or immunosuppressive agents.
Syndromes of neuromuscular hyperactivity
Hyperactivity syndromes, such as Stiff-person syndrome or neuromyotonia (Isaac’s syndrome), are rare but have been associated with malignancies, including small-cell lung cancer, breast cancer, lymphoma, and invasive thymoma . Stiff-person syndrome is a disorder characterized by muscle rigidity and a worsening of symptoms with exposure to certain triggers, such as loud noise or startle. Continuous motor unit activity is noted on needle electromyography, but otherwise electrodiagnostic findings are unremarkable. Antibodies to glutamic acid decarboxylase are present in up to 60% of patients; in some instances there is an association with antibodies against the presynaptic cell membrane protein amphiphysin. Isaac’s syndrome is an autoimmune channelopathy that has been reported in association with Hodgkin’s lymphoma as well as plasmacytoma . Antibodies to voltage-gated potassium channels are present in 50% of patients. Continuous motor unit activity is again noted on needle EMG, however unlike Stiff-person syndrome, the symptoms and findings persist during sleep. Neurotonic discharges may also be present.
Myopathy
The findings of a symmetric, proximal myopathy on clinical examination and electrodiagnostic testing can also lead to the discovery of an undiagnosed cancer. Although their classification as a true paraneoplastic syndrome is controversial, polymyositis, and especially dermatomyositis, are associated with an increased incidence of malignancy compared with the general population . Breast, lung, and gynecologic malignancies are most frequently implicated. Paraneoplastic necrotizing myopathy and carcinoid myopathy are syndromes distinct from polymyositis or dermatomyositis. Carcinoid tumors may be associated with a progressive myopathy that has its onset years after the carcinoid syndrome .
Motor neuron disease
With regard to motor neuron disease syndromes, as mentioned previously the anti-Hu-associated paraneoplastic encephalomyelitis, sensory neuronopathy, and motor neuropathy syndrome has a strong link with small-cell lung cancer. Subacute motor neuropathy and primary lateral sclerosis have been associated with lymphoma and breast cancer, respectively . There is no known association between cancer and amyotrophic lateral sclerosis; however, in newly diagnosed motor neuron disease a screening for cancer is usually part of the exclusionary diagnostic workup.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







