Critical illness myopathy, neuropathy, and neuromyopathy are frequently encountered in the intensive care unit, particularly in the setting of sepsis and the systemic inflammatory response syndrome. A multidisciplinary approach is important to optimize management and minimize debility associated with these neuromuscular disorders. This article reviews the underlying pathophysiology, risk factors, clinical presentation, electrodiagnostic evaluation, management, and prognosis of these disorders.
Severe, generalized weakness is an increasingly recognized complication in patients who have critical illness. Before the advent of modern cardiopulmonary support in the intensive care unit (ICU), high mortality rates precluded clinical recognition of neuromuscular disorders associated with critical illness. In that era, the primary neuromuscular disorders recognized in critically ill patients were those such as myasthenia gravis and Guillain-Barre syndrome, which typically preceded, and occasionally resulted in, sepsis or multiple organ failure. As medical and surgical improvements led to improved survival of patients who have critical illness, a distinct neuromuscular syndrome was recognized and reported . Electrodiagnostic studies revealed a length-dependent, sensorimotor polyneuropathy, and eventually this condition was named critical illness polyneuropathy .
Early electrophysiologic and morphologic studies in patients who had critical illness polyneuropathy also showed abnormalities of muscle fibers , and myopathy associated with critical illness was later recognized in many forms . Ultimately an all-encompassing term, critical illness myopathy , was proposed. Although the relative frequency of critical illness myopathy and polyneuropathy remains the source of some controversy, it is now recognized that many, if not most, patients have electrophysiologic and morphologic evidence of both . Despite reviews that consider the topic of critical illness polyneuropathy and myopathy separately (including this one), it may be more useful for the practicing clinician to approach the weak, critically ill patient as having a possible critical illness neuromyopathy. Such an approach reminds the evaluating clinician to be alert for features of both myopathy and polyneuropathy, which has important implications in terms of prognosis , and for future research studies focusing on pathogenesis and treatment of these disorders.
Approach to the critically ill patient who has limb and respiratory muscle weakness
Neuromuscular manifestations of critical illness are typically first recognized as failure to successfully wean from mechanical ventilation, or as generalized limb weakness. Careful review of past medical history and previous medical records, including collateral history from family and acquaintances, is important to establish the presence of a neurological condition that preceded the development of critical illness. Neuromuscular conditions, such as motor neuron disease, myasthenia gravis, Lambert-Eaton myasthenic syndrome, or Gullain-Barre syndrome, can lead to respiratory failure and pneumonia caused by aspiration, particularly when the respiratory and bulbar muscles are involved. Rapidly progressive acute and subacute infectious or neoplastic disorders, causing myelopathy or polyradiculopathy, need to be considered. Occasionally these disorders elude diagnosis, or progress so rapidly that diagnosis is not possible before admission to the ICU.
A systematic, localization-based approach, considering possible involvement of the brain, spinal cord, peripheral nerves, muscle, or neuromuscular junction is important ( Box 1 ). Septic encephalopathy is an early and very common manifestation of critical illness, occurring in as many as 70% of patients who have critical illness . Encephalopathy in critical illness reflects functional, not structural, disease . The presence of focal signs on examination, such as hemiparesis, asymmetric hyperreflexia, or Babinski signs, should prompt further diagnostic testing, such as head CT or brain MRI, and cerebrospinal fluid testing. Spinal cord imaging with either CT or MRI should also be considered for critically ill patients who have weakness and upper motor neuron signs, such as hyperreflexia and Babinski signs, on examination.
Muscle disorders
Acid maltase disease
Dystrophinopathies
Critical illness myopathy
Polymyositis/dermatomyositis
Neuromuscular junction disorders
Myasthenia gravis
Lambert-Eaton myasthenic syndrome
Botulism
Neuromuscular blocking agents
Neuropathy/motor neuron disorders
Guillian-Barre syndrome
Chronic inflammatory demyelinating polyradiculoneuropathy
Motor neuron disease
West Nile encephalomyelitis
Spinal cord disorders
Ischemia
Hemorrhage
Trauma
Neoplasm
Electrodiagnostic testing, including nerve conduction studies (NCS), needle electromyography (EMG), and repetitive nerve stimulation (RNS), provides the best opportunity to characterize the cause of weakness as a disorder of anterior horn cells, peripheral nerve, muscle, or neuromuscular junction. RNS is used to determine the presence of a neuromuscular junction disorder such as myasthenia gravis, Lambert-Eaton myasthenic syndrome, botulism, or transient neuromuscular blockade following administration of a neuromuscular blocking agent. Serum creatine kinase (CK) determination and muscle biopsy is occasionally used to characterize the nature of a suspected myopathy.
Sepsis, multiple organ failure, and systemic inflammatory response syndrome
Critical illness refers to the syndrome of sepsis and multiple organ failure. Sepsis has historically been defined as a severe, systemic response to infection. The concept of the systemic inflammatory response syndrome (SIRS) was developed to clarify terminology, acknowledging that a severe systemic inflammatory response occurs in noninfectious disorders such as trauma . Sepsis is applied in the SIRS when infection has been documented.
Clinical manifestations of SIRS have been established, including: (1) body temperature of greater than 38°C or les than36°C; (2) heart rate greater than 90; (3) tachypnea, indicated by respiratory rate greater than 20 or PaCo 2 of less than 32; (4) abnormal white blood cell count, either greater than 12,000 cells/mm 3 or less than 4000 cells/mm 3 , or less than 10% “bands” ( Fig. 1 ) .
Humoral and cellular responses are activated in SIRS and sepsis, which produce diffuse microcirculatory changes throughout the body . The humoral response is triggered by epithelial cells, endothelial cells, macrophages and neutrophils, which induce proinflammatory cytokines such as interleukins-1, -2, and -6, tumor necrosis factor (TNF)-α and free radicals . Adhesion molecules adhere to endothelial cells, platelets, and leukocytes, which leads to capillary obstruction and subsequent endothelial damage . Endothelial damage results in tissue edema and promotes a prothrombotic state . The cumulative impact of these changes results in microcirculatory disturbances, which impair energy substrate delivery to end organs, thereby causing organ dysfunction or failure .
Critical illness polyneuropathy develops in 50% to 70% of patients who have sepsis . At least 33% of ICU patients treated for status asthmaticus develop critical illness myopathy , and in patients undergoing liver transplantation, critical illness myopathy develops in 7% . The prevalence of critical illness myopathy in SIRS is unknown.
Sepsis, multiple organ failure, and systemic inflammatory response syndrome
Critical illness refers to the syndrome of sepsis and multiple organ failure. Sepsis has historically been defined as a severe, systemic response to infection. The concept of the systemic inflammatory response syndrome (SIRS) was developed to clarify terminology, acknowledging that a severe systemic inflammatory response occurs in noninfectious disorders such as trauma . Sepsis is applied in the SIRS when infection has been documented.
Clinical manifestations of SIRS have been established, including: (1) body temperature of greater than 38°C or les than36°C; (2) heart rate greater than 90; (3) tachypnea, indicated by respiratory rate greater than 20 or PaCo 2 of less than 32; (4) abnormal white blood cell count, either greater than 12,000 cells/mm 3 or less than 4000 cells/mm 3 , or less than 10% “bands” ( Fig. 1 ) .
Humoral and cellular responses are activated in SIRS and sepsis, which produce diffuse microcirculatory changes throughout the body . The humoral response is triggered by epithelial cells, endothelial cells, macrophages and neutrophils, which induce proinflammatory cytokines such as interleukins-1, -2, and -6, tumor necrosis factor (TNF)-α and free radicals . Adhesion molecules adhere to endothelial cells, platelets, and leukocytes, which leads to capillary obstruction and subsequent endothelial damage . Endothelial damage results in tissue edema and promotes a prothrombotic state . The cumulative impact of these changes results in microcirculatory disturbances, which impair energy substrate delivery to end organs, thereby causing organ dysfunction or failure .
Critical illness polyneuropathy develops in 50% to 70% of patients who have sepsis . At least 33% of ICU patients treated for status asthmaticus develop critical illness myopathy , and in patients undergoing liver transplantation, critical illness myopathy develops in 7% . The prevalence of critical illness myopathy in SIRS is unknown.
Critical illness polyneuropathy
Patients who have critical illness polyneuropathy develop distal weakness, may have depressed deep tendon reflexes, and not uncommonly fail to wean from mechanical ventilation. Sensory loss may be difficult to demonstrate, given the high prevalence of encephalopathy in critical illness; however, in a patient with spontaneous limb movements, failure to withdraw a limb following administration of painful stimulation to the distal limb suggests sensory loss. Cranial nerve abnormalities are exceedingly rare and should suggest an alternative diagnosis.
Electrodiagnostic features
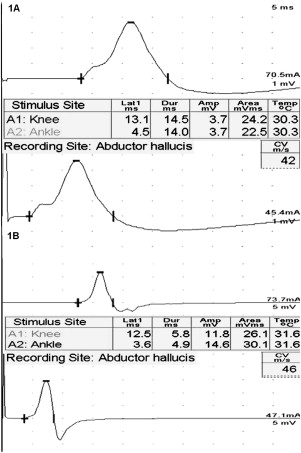
Thoughtful electrodiagnostic testing is important in the evaluation of suspected critical illness polyneuropathy. Upper and lower limb motor and sensory NCS are performed. In patients who have suspected respiratory muscle failure, phrenic NCS are also performed. RNS is normal in critical illness polyneuropathy, and is helpful to exclude a pre-existing neuromuscular junction disorder or weakness caused by transient neuromuscular blockade following administration of a neuromuscular junction blocking agent. Because the majority of patients are too weak to exercise, brief, high-frequency repetitive stimulation (at 20 or 50 Hz x 1–2 seconds) should be performed to evaluate for significant facilitation of the compound muscle action potential amplitude. This is particularly important if low-amplitude compound muscle action potentials (CMAP) are present with routine NCS, typically seen in the Lambert-Eaton myasthenic syndrome. Motor CMAP amplitudes and sensory nerve action potential (SNAP) amplitudes are typically reduced, with normal or near normal conduction velocities and distal latencies. Decline in the CMAP amplitudes occurs early in the course of the illness, and may be followed by subsequent decline in the SNAP amplitudes later . Significant conduction velocity slowing or distal latency prolongation and features of temporal dispersion or conduction block should suggest an alternative diagnosis, such as Guillain-Barre syndrome.
Needle EMG typically shows abnormal spontaneous activity in distal muscles, and may show reduced recruitment of motor unit potentials. The presence of small motor unit potentials on needle EMG should alert the electromyographer to the possibility of a concomitant myopathy, particularly if the SNAP amplitudes are normal or near-normal. Single-fiber EMG studies in nine patients who had critical illness polyneuropathy showed increased jitter, suggesting a disorder of nerve terminals in these patients ; however, assessment of motor unit potentials can be difficult in the encephalopathic patient, who may be unable to activate motor unit potentials. In a patient who has low-amplitude CMAPs and preserved SNAPs, and who is unable to activate motor unit potentials, it may not be possible with routine electrodiagnostic studies to determine whether the patient has a myopathy, polyneuropathy, or both. Prolonged CMAP duration and muscle inexcitability on direct muscle stimulation suggests the presence of myopathy. In such a patient, repeat NCS and needle EMG should be considered following improvement in encephalopathy or weakness.
Pathology and pathophysiology
Nerve biopsy and postmortem autopsy studies showed evidence of primary axonal degeneration, without findings of inflammation or primary demyelination . Axonal degeneration of intercostal and phrenic nerves and denervation atrophy in respiratory muscles was felt to explain the respiratory insufficiency in these patients .
The cause of critical illness polyneuropathy is speculative. Many different factors have been hypothesized in the pathogenesis of critical illness polyneuropathy. No drug, toxin, infection, nutritional deficiency, or iatrogenic agent has been identified as being causative. It is likely that systemic physiologic changes associated with sepsis, multiple organ failure, and the SIRS cause critical illness polyneuropathy. Critical illness neuropathy severity has been associated with ICU length of stay, elevated serum glucose levels, and decreased serum albumin levels . Critically ill patients who have high APACHE (Acute Physiology, Age, Chronic Health Evaluation)-III score and the SIRS are most prone to the development of critical illness neuropathy .
The current, prevailing hypothesis is that cytokines secreted in sepsis increase microvascular permeability , resulting in endoneural edema, leading to failure of distal axonal transport and subsequent axonal degeneration . The lack of peripheral nerve microvascular autoregulation likely enhances susceptibility to such a process . The role of a neurotoxin in critical illness neuropathy has been suggested but not definitively demonstrated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







