Chapter 29 Neurogenic Bowel
Dysfunction and Rehabilitation
Gastrointestinal dysfunction is most often characterized by a conglomeration of symptoms that most often indicate lower gastrointestinal impairment, including constipation, diarrhea, and fecal incontinence (FI). It can also present as upper gastrointestinal impairment heralded by bloating, nausea, early satiety, burning, and gaseousness. Neurogenic bowel dysfunction can be a clinically elusive impairment. It is often eclipsed by other, more noticeable associated motor deficits. Neurogenic bowel dysfunction itself can be particularly life-limiting if it is not thoroughly assessed and treated using rehabilitation principles. Interdisciplinary rehabilitative interventions focus on establishing a total management plan for bowel function, termed a bowel program, and for assisted defecation, known as bowel care.107 Sensation and mobility might be limited, affecting a person’s ability to anticipate the need for and to physically perform independent bowel care and hygiene.
In spite of many abilities regained during the rehabilitation process, bowel care capabilities at the time of discharge are not always comparable to other skills that would be expected for a given level of function. Bowel incontinence is one of the greatest predictors of return to home for stroke survivors.29 In fact, bowel management has been found to be one of the areas of least competence among rehabilitated persons with spinal cord injury (SCI).14,50 More than one third of surveyed persons with SCI rated bowel and bladder dysfunction as having the most significant effect on their lives after SCI.51 In a recent Swedish review of medical problems after SCI, 41% of subjects rated bowel dysfunction as a moderately to severely life-limiting problem.74
Epidemiology
Gastrointestinal dysfunction is frequently seen in persons with neurologic diseases who require rehabilitation. Besides the direct effects of neurologic disease on gut function, other factors can also play a huge role in the development of enteric problems, including debility, insufficient fluid intake, and the use of anticholinergics and other medications. Digestive tract problems in stroke, brain injury, multiple sclerosis, Parkinson’s disease, neuromuscular diseases, dysautonomias, peripheral nerve injuries, SCIs, and other neurologic disorders have been shown to be difficult and challenging to manage. Neurogenic bowel difficulties can be a primary disabling and handicapping feature for patients with SCI, stroke, amyotrophic lateral sclerosis, multiple sclerosis, diabetes mellitus, myelomeningocele, and muscular dystrophy.∗
Neurogenic bowel dysfunction results from autonomic and somatic denervation, and produces FI, constipation, and difficulty with evacuation (DWE). These symptoms are common. The prevalence of FI and fecal impaction ranges from 0.3% to 5.0% in the general population. The prevalence of DWE ranges from 10% to 50% among the hospitalized or institutionalized elderly.100,115 Although many gastrointestinal disorders can contribute to FI or DWE, disorders that impair the extrinsic (sympathetic, parasympathetic, or somatic) nervous control of the bowel and anorectal mechanisms are more common among the patient populations seen by physiatrists.
Impact
FI decreases the return-to-home rates for stroke patients.49 Almost one third of persons with SCI report or exhibit worsening of bowel function 5 years beyond their injury, with 33% developing megacolon, suggesting inadequate long-term management.52,110 Recent evidence has shown some improvement in outcomes for SCI bowel management.67 When restoring normal defecation is not possible, social continence becomes the goal. Social continence is defined as predictable, scheduled, adequate defecations without incontinence at other times. It is often achievable by persons with neurogenic bowel dysfunction. Embarrassment and humiliation from FI frequently result in extreme vocational and social disability. Vocational disability and excessive institutionalization add substantial costs to the care related to neurogenic bowel dysfunction. Nursing home costs are higher for patients with FI.115 A 1983 report estimated that $8 billion per year is spent in the United States for the care of fecally incontinent institutionalized patients.100
Neuroanatomy and Physiology of the Gastrointestinal Tract
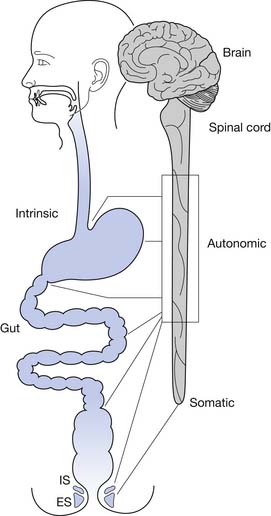
Normal functioning of the stomach and intestines entails coordination of muscle contraction, digestion and absorption of nutrients, and regulation of blood flow. The neural schema of the gastrointestinal tract is much more complex than previously thought. Neural control of the gastrointestinal tract is an extremely organized and integrated hierarchy of mechanisms that involve the central nervous system (CNS; brain and spinal cord), autonomic nervous system (sympathetic and parasympathetic), and enteric nervous system (ENS) (Figures 29-1 and 29-2).123,126
Enteric Nervous System
The ENS is a distinct system that has its own set of neurons that coordinate sensory and motor functions. In the ENS the ganglia are interconnected, which allows for integration and processing of data (as opposed to autonomic ganglia, which only serve as relay centers for stimuli transmitted from the CNS). There are three different types of neurons in the ENS based on function: sensory neurons, interneurons, and motor neurons.123,126 Sensory neurons perceive thermal, chemical, or mechanical stimuli and transform these sensations into action potentials that are conducted to the nervous system. Interneurons serve as conduits between the sensory and motor neurons. The numerous synapses between interneurons create a highly organized circuitry that processes sensory input from the gut and other parts of the nervous system, and integrates and generates reflex responses to these stimuli. Motor neurons are the final common pathway. They receive and translate signals to the gut (mucosa, muscle, vasculature) that affect digestive, interdigestive, and emetic functions based on the transmitters released.123,126
Automatic feedback control is present in the ENS, with the neurons being in close proximity to the stomach and intestines. This can be manifested as reflex circuits that systematize reflex responses to sensory signals, as integrative circuits that coordinate motor patterns (migrating motor complex, digestive activity, giant migratory contractions),123,126 or as a pattern-generating activity occurring when a “command neuron” is actuated with a resulting rhythmic, repetitive behavior.11,126
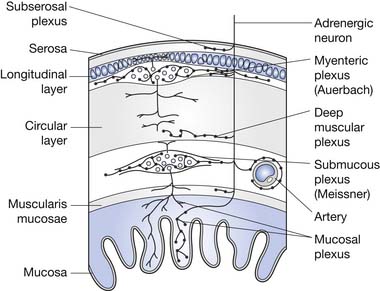
The ENS is the key to proper functioning of the entire gastrointestinal tract. This collection of highly organized neurons is situated in two primary layers: the submucosal (Meissner’s) plexus and the intramuscular myenteric (Auerbach’s) plexus. These plexi have an estimated 10 to 100 million neurons, plus two to three glial cells per neuron. The ENS glial cells resemble CNS astrocytes and are much less abundant than the 20 to 50 glial cells per neuron in the CNS.46 The coordination of segment-to-segment function is largely regulated by the ENS.121 The ENS also has its own blood-nerve barrier, similar to the blood-brain barrier of the CNS.24
Gastrointestinal Neurosensory System
Enteric Nervous System Sensory Neurons
The ENS relays chemical, mechanical, and thermal sensory information to the CNS through vagal afferents and spinal afferents. Vagal and spinal sensory neuron endings supply the muscle, mucosa, and ganglia of the ENS. Spinal sensory neurons also supply the serosa and mesentery and their blood vessels.11,47
Vagal afferent nerve endings act as chemical, thermal, and mechanical receptors. These directly monitor various changes in the gastrointestinal tract such as chemical milieu, temperature, muscle tension, and mucosal brushing.11,47
Chemical receptors are located in the mucosal epithelium of the stomach and intestinal lumen in close proximity with the lamina propria, constantly determining acidity, osmolarity, and the concentration of glucose, fatty acids, and amino acids.11,47 The existence of nociceptors (C or Aδ fibers) in the gastrointestinal tract has not been established.21,22,126 Temperature change in the lumen is detected by thermoreceptors that play a role in the brain’s perception and regulation of core temperature.126
Mechanical receptors are classified as intramuscular arrays or intraganglionic laminar endings. Intramuscular arrays are adjacent to the intramuscular interstitial cells of Cajal and run contiguously with the long muscle layers.43,47 Intraganglionic laminar endings that follow circular and longitudinal muscle fibers provide data on distention or contraction, and connect with myenteric ganglia.47,129 They are also found in rectal muscles and contribute to pelvic innervation.47,78
Spinal afferent nerve endings are distributed extensively throughout the bowel, relaying chemical, thermal, and mechanical information. In the mucosa they detect chemical changes related to injury, ischemia, infection, or inflammation that contribute to pain and discomfort.47,66 Spinal nerve endings express receptors for bradykinin, adenosine triphosphate (ATP), adenosine, prostaglandins, leukotrienes, histamine, mast cell proteases, and serotonin 5-hydroxytryptamine3 (5-HT3). In the serosa and mesentery, they recognize visceral distention and contraction.70,82,83 Those that innervate the blood vessels and ENS ganglia release neurotransmitters that affect gastrointestinal blood flow, motility, and secretory reflexes.47,83
Mechanoreceptors (whether derived from vagal or spinal afferents) are either low or high in threshold.101 Low-threshold receptors detect normal nonconscious and conscious sensations such as satiety, hunger, gaseousness, and nausea. High-threshold receptors detect distention or contraction beyond a set threshold and perceive painful stimuli, eliciting acute, sharp visceral pain. Actuation of both low- and high-threshold receptors contributes to the range of symptoms that are felt along the gastrointestinal tract.21,22,44,47 In the setting of inflammation or ischemia, chronic pain can develop from sensitization of both types of receptors and activation of silent receptors (notable only in pathologic states). Vagal afferents are primarily implicated in the emetic response, and spinal afferents are primarily implicated in the sensation of nausea.44,47
Enteric Nervous System Relationship to the Spinal Cord and Brain
Sensory information from vagal afferents in the ENS is relayed to the nodose ganglia (caudal ganglion of the vagus) and consequently to the nucleus tractus solitarius (NTS) and area postrema in the medullary area of the brainstem. The NTS and the area postrema send signals to the rostral centers in the brain.123,126 The brain processes the data and projects descending connections to the dorsal vagal complex. The brain also participates in vago-vagal reflex circuits that continuously monitor and promptly modify responses to changes in the chemical, thermal, and mechanical environment in the whole gut (mostly in the esophagus, stomach, duodenum, gallbladder, and pancreas). Sensory stimulation through this vagal circuit does not appear to reach the level of consciousness.123,126
Besides making connections with the NTS and the area postrema in the brainstem, vagal afferents synapse with the dorsal motor nucleus of the vagus and the nucleus ambiguus, creating the dorsal vagal complex. The incoming sensory information is integrated and shared with the forebrain and brainstem by the dorsal vagal complex. Brain coordination and influences (conscious and nonconscious) are translated to the dorsal vagal complex, where the dorsal motor nucleus of the vagus and nucleus ambiguus represent the efferent arm of the reflex pathway. It is the final common pathway from the brain responsible for precise control of muscular, glandular and circulatory responses of the gastrointestinal tract.123,124,126 Multiple neurotransmitters are involved in the intricate conduction of impulses between the neuronal circuits in the dorsal vagal complex. Approximately 30 are identified and include acetylcholine, biogenic amines, amino acids, nitric oxide, and peptides.123,126
Spinal afferents (splanchnic and pelvic) send sensory impulses to the dorsal root ganglia (or prevertebral sympathetic ganglia) that are then conducted to the dorsal horn (laminae I, II, V, X) of the spinal cord and the dorsal column nuclei.47,88 The dorsal column is thought to play a greater role in nociceptive transmission of messages from the gut than the spinothalamic or spinoreticular tracts. The conscious perception of pain in the digestive tract is largely mediated through spinal afferents. The spinal cord modulates the conduction of neural messages (both nociceptive and nonnociceptive) to the higher brain.47 Other somatic and visceral sensory stimuli from the vagina, uterus, bladder, colon, and rectum are conveyed through the dorsal horn and dorsal column in the spinal cord as well. Likewise, somatic afferents supply sensory input from the muscles of the pelvic floor through the pudendal nerve to the sacral region of the spinal cord.10,47,88,89
Brain centers modulate the nociceptive impulses from the bowel that are relayed to the dorsal horn of the spinal cord. These descending pathways are facilitatory, inhibitory, or both, based on the visceral stimulus, and can alter the perception of pain in the digestive tract.47,97 The neurotransmitters serotonin, noradrenalin, and dopamine are released by these descending pathways as they synapse with the spinal cord.126
The secondary somatosensory cortex and, to a lesser extent, the paralimbic and limbic areas (anterior insular, anterior and posterior cingulate, prefrontal and orbitofrontal cortices) mediate emotional, volitional, and psychologic responses to sensory input from the gut. These are manifested by abdominal pain, anorexia, nausea, vomiting, hyperphagia, constipation, or diarrhea.47,73,104 The somatosensory cortex regulates the awareness and recognition of pain, and the paralimbic and limbic areas contribute to the cognitive and affective aspects of pain.47,104,126
Data from the brain and descending vagal pathways are conducted to the spinal cord, and then with the preganglionic neurons in the thoracolumbar area (modulates the sympathetic response) and the sacral area (modulates the parasympathetic response). Sympathetic or parasympathetic output is translated to the neurons in the ENS circuitry, which can be excitatory or inhibitory to motor neurons, gastric or digestive glands, and secretory mechanisms.47,126
Gastrointestinal Neuromotor System
The muscles of the gastrointestinal tract carry out essential functions throughout the gut, including propulsion, grinding, mixing, absorption, storage, and disposal. These muscles are composed of “self-excitable” smooth muscles that contract in an all-in-one manner. These smooth muscles spontaneously responds to stretch and can be independent of neural or endocrine control. The interstitial cells of Cajal act like a pacemaker and allow propagation of electrical slow waves into the circular muscle layer, which generates spreading muscle contraction. These smooth muscles act like an electrical syncytium where action potentials are conducted in three dimensions from one smooth muscle fiber to another through gap junctions.47,126
The gastrointestinal tract muscles respond to influences of the vagal efferents and the ENS microcircuitry based on excitatory or inhibitory innervation of motor neurons. Contraction is mediated by the release of excitatory neurotransmitters by vagal afferents at the neuromuscular junctions, acetylcholine (at the muscarinic receptor), and substance P (at the neurokinin-1 receptor).26,47,82,126 Conversely, the release of nitric oxide, ATP, and vasoactive intestinal peptide from the inhibitory motor neurons (which express the serotonergic 5-HT1 receptor) impedes contractile activity and facilitates relaxation. The aboral direction of propulsive activity throughout the digestive tract is achieved by segmental inactivation of inhibitory motor neurons distally.15,16,47,58,126 Contraction can only occur in the segments where the inhibitory motor neurons are inactivated. With passing of the food bolus or stool, the esophageal and internal anal sphincters (smooth muscle sphincters), and inhibitory motor neurons are usually shut off and are inactivated. During vomiting, however, the inhibitory motor neurons are deactivated in the opposite direction.47,126
The sympathetic and parasympathetic nervous systems seem to modulate the ENS, rather than directly controlling the smooth muscles of the bowel.121 The smooth muscles of the bowel also have their own electromechanical automaticity, which is directly modulated by the inhibitory control of the ENS.24,46 Sympathetic nervous system stimulation tends to promote the storage function by enhancing anal tone and inhibiting colonic contractions, although little clinical deficit occurs from bilateral sympathectomy.32 Parasympathetic activity enhances colonic motility, and its loss is often associated with DWE, including impactions and functional obstructions, such as Ogilvie’s pseudoobstructive syndrome.32
The ENS and sympathetic postganglionic neurons transmit excitatory or inhibitory messages to secretomotor neurons. These secretomotor neurons release acetylcholine and vasoactive intestinal polypeptide when there is excitatory activation from paracrine stimulation by mucosal and submucosal cells such as enterochromaffin cells, mast cells, and other immune and inflammatory cells. Acetylcholine and vasoactive intestinal polypeptide are released at the neuroepithelial and neurovascular junctions. This promotes secretion into the gut of water, sodium chloride, bicarbonate, and mucus drawn from the intestinal glands. In addition, dilatation and an increase in blood flow occur with the release of nitric oxide from the vascular endothelium.6,13,47 The inhibitory influence reduces neuronal firing from secretomotor neurons by hyperpolarization of membranes. Sympathetic release of norepinephrine from nerve endings of the α2-noradrenergic receptors inhibits secretomotor actuation, preventing the release of excitatory neurotransmitters. As a result, there is a decrease in secretion of water and electrolytes into the lumen and a congruent shunting of blood from the splanchnic to systemic circulation.47,76,91
Gastric Motility
Based on its motility pattern the stomach is divided into an upper and lower portion. The upper portion (fundus) has sustained, low-frequency contractions and has a tonic pattern. The lower portion (antrum) has intermittent, powerful contractions and has a phasic pattern. The fundus acts as reservoir and accommodates incoming food, which inhibits contraction and allows the stomach to stretch without a significant increase in pressure. The antrum is a mixer that generates propulsive waves that accelerate as food is propagated towards the pylorus. The amount and consistency of food in the fundus regulates excitatory and inhibitory influences and adjustments to volume and pressure.47,69
Intestinal Motility
The ENS is designed to control the various patterns of motility in the intestinal tract. The interdigestive migrating motor complex pattern occurs during fasting in the stomach and the small intestine.47,120 It seems to be influenced by the hormone motilin, and is responsible for removal of waste from the intestinal lumen throughout the fasting period.47,77 When a meal is ingested, the postprandial segmentation (“mixing”) pattern of motility commences as digestion transpires. The brainstem sends signals that are transmitted to vagal efferents, which convert migrating motor complex motility to segmentation motility with the increase in bulk and nutrients, especially lipids (medium-chain triglycerides). This subsequently becomes peristaltic motility, which is propagated through brief segments of intestine at a time.30,47 Peristaltic activity gradually evolves into powerful contractions sustained through long portions of circular muscle along the small and large bowel. These “giant migratory contractions” (GMCs) propel waste through the lumen, particularly in the large intestine.47,59,99
Motility of the Anus, Rectum, and Pelvic Floor
Normal defecation and maintenance of fecal continence entail a highly coordinated mechanism that involves the levator ani, puborectalis, and the external (EAS) and internal anal sphincter (IAS) muscles. The pelvic floor is composed of the levator ani, the underlying sheets of which form a sling. The levator ani, puborectalis, and EAS are skeletal muscles that constantly maintain tone and sustain pelvic organs in place against the forces of gravity.35,47 Simultaneous contraction of these muscles prevents the involuntary loss of stool and helps maintain the regular pattern of defecation.41,47
Physiology of Normal Defecation
The colon is a reservoir for food waste until it is convenient for elimination. It also acts as a storage device as long as the colonic pressure is less than that of the anal sphincter mechanism. Fecal elimination occurs when colonic pressure exceeds that of the anal sphincter mechanism. Other functions of the colon are to reabsorb fluids (up to 30 L/day can be reabsorbed from the large and small bowel walls, with typically only 100 mL of water loss in feces) and gases (90% of the 7 to 10 L of gases produced by intracolonic fermentation is absorbed rather than expelled). The colon also provides an environment for the growth of bacteria needed to assist in digestion, and serves to absorb certain bacterial breakdown products as well.48 The layers of the colon wall are depicted in Figure 29-3.
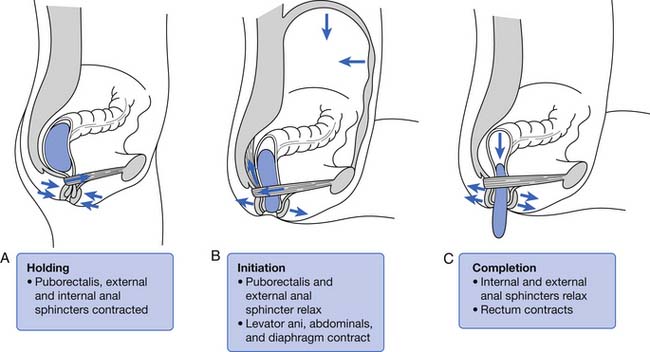
The rectum is usually empty until just before defecation. Perception of rectal contents and pressures105 is essential for signaling voluntary contraction of the anal sphincter. Normal defecation begins with reflexes triggered by rectosigmoid distention produced by approximately 200 mL of feces (Figure 29-4).94 A rectorectal reflex occurs in which the bowel proximal to the distending bolus contracts and the bowel wall distally relaxes, serving to propel the bolus further caudad. Reflex relaxation of the IAS also occurs, which is enhanced by, but does not require, an extrinsic nerve supply. This relaxation, called the rectoanal inhibitory reflex, correlates with the urge labeled “the call to stool.”121 One can then volitionally contract the levator ani to open the proximal anal canal and relax the EAS and puborectalis muscles. This allows a straighter, shorter, and more open anorectal passage (see Figure 29-4), which permits the bolus to pass. Increasing the intraabdominal pressure by squatting and by Valsalva’s maneuver assists bolus elimination. For 90% of normal individuals, only the contents of the rectum are expulsed, whereas 10% will clear the entire contents of the left side of the colon from the splenic flexure distally.33
One can elect to defer defecation, however, by volitionally contracting the puborectalis muscle and EAS. The reflexive IAS relaxation subsequently fades, usually within 15 seconds, and the urge resolves until the IAS relaxation is again triggered. The rectal wall accommodates to the bolus by decreasing its wall tension with time, resulting in less sensory input and less reflex triggering from that particular accommodated bolus. This continence and reflex process is somewhat analogous to the function of the striated external urethral sphincter in volitional control of urinary voiding (see Chapter 28).
The EAS generally tenses in response to small rectal distentions via a spinal reflex, although reflexive relaxation of the EAS occurs in the presence of greater distentions.105 These spinal cord reflexes are centered in the conus medullaris and are augmented and modulated by higher cortical influences. When cortical control is disrupted, as by SCI, the EAS reflexes usually persist and allow spontaneous defection. During sleep the colonic activity, anal tone, and protective responses to abdominal pressure elevations are all decreased, while rectal tone increases.24,121
The gastrocolonic response or gastrocolic reflex refers to the increased colonic activity (GMCs and mass movements) in the first 30 to 60 minutes after a meal. This increased colonic activity appears to be modulated both by hormonal effects, from release of peptides from the upper gastrointestinal tract (gastrin, motilin, cholecystokinin) that increase the contractility of the colonic smooth musculature, and by a reduction in the threshold for spinal cord–mediated vesicovesical reflexes.24 Upper gastrointestinal receptor stimulation also results in increased activity in the colon, possibly because of reflexively increased parasympathetic efferent activity to the colon. The possibility of a purely ENS-mediated activation exists, although the small bowel and colon motor activities do not seem to be synchronized. In persons with SCI the measured increase in colonic activity after a meal is blunted as compared with that in normal subjects.24 The gastrocolonic response is often used therapeutically, even in patients with SCI, to enhance bowel evacuation during this 30- to 60-minute postprandial time frame.1,34 Occasionally certain foods can serve as trigger foods that are especially likely to induce bowel evacuation shortly after consumption.
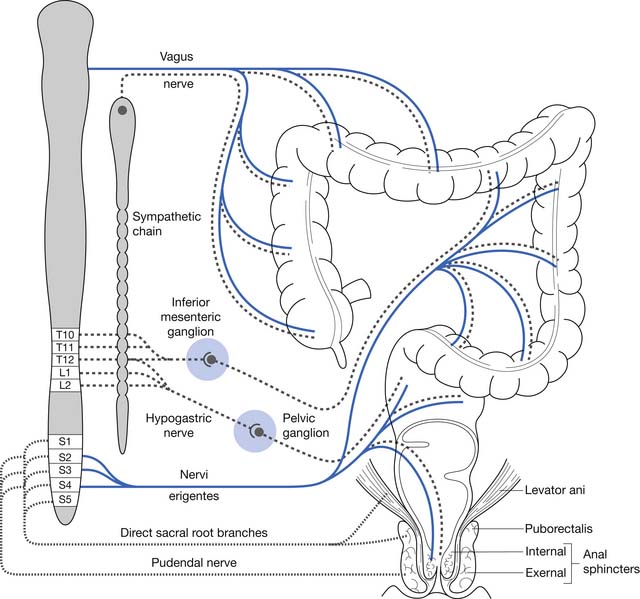
The resting anal canal pressure is largely determined by the angulation and pressure at the anorectal junction by the puborectalis sling and smooth muscle IAS tone. Continence is maintained by the anal sphincter mechanism,81,107 which consists of the IAS, EAS, and puborectalis muscle.81 Only about 20% of the anal canal pressure is due to the static contraction of the somatically innervated striated EAS.9 The EAS and puborectalis muscle are the only striated skeletal muscles whose normal resting state is tonic contraction, and these muscles consist mainly of slow-twitch, fatigue-resistant type I fibers (unlike the situation in nonupright animals such as the cat or dog, in which these muscles consist of predominately type II fibers).9 Anal pressure can be increased volitionally by contracting the EAS and puborectalis muscles. Maximum volitional squeeze pressures, however, are not as high as can be generated reflexively against Valsalva pressure. The EAS is physically larger than the IAS, and its contraction is under both reflex and volitional control. The volitional control is learned during the course of normal maturation. Normal baseline reflex action of the anorectal mechanism allows spontaneous stool elimination.102 The EAS is innervated by the S2 through S4 nerve roots via the pudendal nerve, and the puborectalis muscle is innervated by direct branches from the S1 to S5 roots (see Figures 29-1 and 29-2).92 The remarkable degree of learned EAS coordination allows the selective discrete passage of gas while juggling a variable mixture of solids, liquids, and gases.
Pathophysiology of Gastrointestinal Dysfunction
A whole range of neurologic diseases affecting central, peripheral, and intrinsic enteric nervous innervation can cause disorders that affect various segments of the bowel. These are predominantly characterized by disturbances in gastroesophageal, small or large intestinal motility and by disturbances in sensation. Symptoms of dysphagia, vomiting, bloating, abdominal discomfort and pain, constipation, and incontinence have been described in individuals with neurologic ailments.5,18,19,93
Nausea, Vomiting, and Bloating
The syndrome of nausea, vomiting, bloating, and early satiety in the setting of neurologic conditions without mechanical obstruction can herald motility problems in the gastrointestinal tract. Neurologic dysfunction that affects the inhibitory motor neurons in the ENS at any level of the neural axis from the brain, spinal cord, afferent nerves, or efferent nerves can lead to spasticity of the gastric or intestinal and colonic musculature. The digestive muscles perform as an electrical syncytium. Inhibitory motor neurons allow propagation of contractile activity in an organized, segmental, and aboral pattern. When inhibitory motor neurons are inactivated or destroyed by disease, the circular muscles contract continuously and nonsystematically. These contractions are incapable of forward propulsion, causing functional obstruction.123,125 This can be manifested as dysphagia, gastroparesis, or chronic intestinal or colonic pseudoobstruction, which might be associated with anorexia, abdominal pain, and diarrhea and constipation (these symptoms can occur together). Inhibitory motor neurons can be affected by autonomic neuropathy, dysfunction of neurons in the myenteric plexus, or degeneration of smooth muscle.∗
Abdominal Pain and Discomfort
Abdominal pain and discomfort arise from gastrointestinal tract distention and powerful contractions. High-threshold and silent mechanoreceptors sense severe distention and intense contractions when there is ischemia, injury, or inflammation. Mechanical and chemical irritants stimulate mechanoreceptors in the ENS and translate signals to the brain and spinal cord from muscle stretching and contractions.11,89,125
Diarrhea
Neurologic dysfunction can present with frequent passage of watery stools. Diarrhea occurs when there is overstimulation of secretomotor neurons by histamine from inflammatory and immune-mediated cells in the mucosa and submucosa, or overstimulation by vasoactive intestinal peptide and serotonin from mucosal enterochromaffin cells, or overstimulation by all three substances. Moreover, these substances influence presynaptic inhibitory receptors to block the release of norepinephrine from the postganglionic sympathetic fibers that inhibit secretomotor neurons.124,125 Bacterial overgrowth in the gut can be a factor in chronic inflammatory states presenting with diarrhea.7,27
Defecation Dysfunction
Constipation
Constipation can be a huge enigma in neurologic states. Infrequent, incomplete emptying of hard stools is due to decreased water and electrolyte secretion into the lumen, resulting from reduced excitation of the secretomotor neurons in the ENS. Norepinephrine released by sympathetic stimulation inhibits the firing of secretomotor neurons by hyperpolarization. Release of excitatory neurotransmitters is reduced in the secretory epithelium, decreasing the secretion of water and electrolytes.125,126 A lack of rectal sensation and a decreased urge to defecate can be strongly associated with constipation in various conditions that present with lesions in the brain, spinal cord, sacral nerves, and hypogastric and pudendal nerves. Outlet obstruction can ensue because of delayed colonic transit times and lack of perineal and rectoanal sensation.125
Fecal Incontinence
True FI, described as an unconscious loss of stool, often occurs in neurologic conditions with lesions affecting the lumbar spinal cord, cauda equina, S2–S4 nerves, pudendal nerve, and pelvic floor nerves. Denervation leads to impaired perineal and rectoanal sensation, aberrant contraction, loss of tone, and weakening of the pelvic floor muscles and the EAS. These contribute to unexpected loss of stool and abnormal defecation, and diminished support for pelvic structures.25,47 Parasympathetic augmentation can occur and might further complicate matters, since it contributes to weakness in the IAS and increases the risk for incontinence. It is always important to rule out overflow incontinence resulting from constipation.
Upper Motor Neurogenic Bowel
Any destructive CNS process above the conus, from SCI to dementia, can lead to the upper motor neurogenic bowel (UMNB) pattern of dysfunction. Spinal cortical sensory pathway deficits lead to a decreased ability to sense the urge to defecate. Most persons with SCI, however, sense a vague discomfort when excessive rectal or colonic distention occurs. It has been reported that 43% of persons with SCI have chronic complaints of a vague discomfort caused by abdominal distention that eases with bowel evacuation.80,110 These sensations might be mediated by autonomic nervous system afferent fibers bypassing the zone of SCI via the paraspinal sympathetic chain, or by means of vagal parasympathetic afferents.
Colonic compliance and sphincter tone102 have been experimentally evaluated in subjects with SCI. Studies of colonic compliance in response to a continuous infusion of saline initially suggested rapid pressure rises and a hyperreflexic response.87,117 More recent studies have demonstrated normal colonic compliances in subjects with SCI who have UMNB.79,90 Passive filling of the rectum leads to increases in the resting sphincter tone.114 These increases are associated with increased EAS pressure development resulting from sacral reflexes that can be abolished by pudendal block.9 This form of rectal sphincter dyssynergia has unfortunately been labeled decreased colonic compliance, even though intermittent or slow filling in the rectum appears to be associated with normal bolus accommodation and pressure relaxation.79,100 This contrasts with “true” decreased rectal wall compliance caused by fibrosis resulting from ischemia or inflammation, where there is no accomodation and relaxation of the rectal wall regardless of flow rates.
Colonic motility and stool propulsion are known to be affected by SCI. De Looze et al.29 used a questionnaire method to study subjects with SCI levels above L2, and found 58% of subjects with chronic SCI had constipation (defined as two or less bowel movements per week or the requirement for digital evacuation). Only 30% (p = 0.002) of patients with paraplegia below T10 and above L2, however, were prone to constipation. Actual stool propulsion was studied later by Krogh et al.71 using swallowed markers and serial radiographs. In subjects with chronic SCI with supraconal lesions, transit times were significantly prolonged in the ascending, transverse, and descending colon and rectosigmoid. Total gastrointestinal transit time averaged 3.93 days (control subjects, 1.76 days) for chronic complete SCI above the conus. In an attempt to demonstrate a difference that might have been conferred by sympathetic innervation, mean total gastrointestinal transit times were compared in patients with lesions above T9 (2.92 ± 2.41 days) and in those with lesions from T10 to L2 (2.84 ± 1.93 days). No significant differences could be found even with comparison of transit times for individual colonic segments.
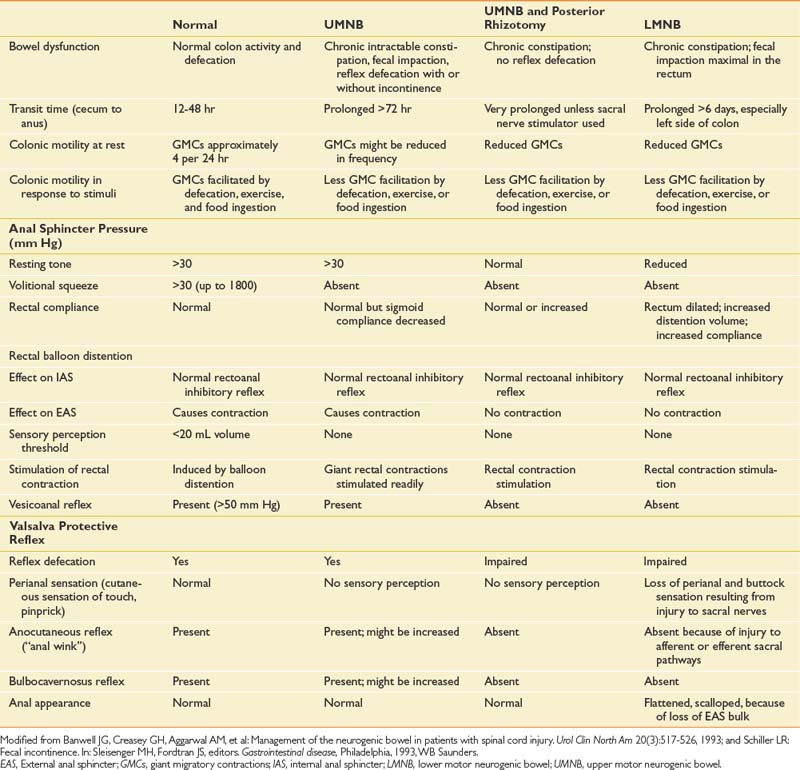
Subjects with SCI who had complete upper motor neuron bowel lesions were studied during the acute phase (5 to 21 days) after SCI. These same patients were reevaluated 6 to 14 months later. Total gastrointestinal transit time was longer during the acute rather than the chronic phase. The upper motor neuron neurogenic colon tended to have slower transit throughout the colon, with less severe rectosigmoid dysfunction.71 Patients with UMNB have spared reflex arc control of the rectosigmoid and pelvic floor. IAS relaxation on rectal distention occurs in persons with SCI as well as in neurologically intact persons. Sufficient rectal distention might cause the EAS to completely relax, resulting in expulsion of the fecal bolus. Rectal sphincter dyssynergia does not necessarily correlate with bladder sphincter dyssynergia, but it often results in DWE.92 The protective vesicorectal reflex, in which the EAS pressure increases in response to increased intraabdominal pressure, is usually intact (Table 29-1).9 Patients with UMNB also have normal or increased anal sphincter tone, intact anocutaneous (or “anal wink”) and bulbocavernosus reflexes,107 a palpable puborectalis muscle sling, and normal anal verge appearance(Figure 29-5).
Table 29-1 Features of Colorectal Function in Normal Subjects and in Those With UMNB, UMNB With Posterior Rhizotomy, and LMNB
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree