Chapter 50 Myositis Ossificans of the Elbow
Introduction
According to the World Health Organization classification of tumours myositis ossificans is:
A non-neoplastic condition, sometimes associated with trauma. The lesion may occur on the external surface of a bone or in soft tissue at a distance from the periosteal surface. The abnormal tissue is characterized by proliferation of fibrous tissue and by formation of large amounts of new bone. Cartilage may also be present.1
In its rare, autosomal dominant form, fibrodysplasia ossificans progressiva (FOP), it is characterized by skeletal malformation and progressive, disabling heterotopic osteogenesis. The condition was first described by Guy Patin in 16922 and in 1868 it was named by Theodore von Dusch3 (1824–1890). Because muscle is not always involved and an inflammatory reaction is not always present, the term ‘myositis’ is perhaps misleading and the alternative nomenclature of heterotopic ossification or ‘extraosseous localized, non-neoplastic bone and cartilage formation’4 is preferable. Others, however, do not agree as heterotopic ossification is defined by some as the formation of lamellar bone inside soft tissue structures where bone normally does not exist, whereas myositis ossificans refers to a condition in which ectopic bone is formed within muscles and other soft tissues. This is differentiated from ectopic calcification, which is the mineralization of soft tissue structures that usually follows chemical or physical trauma, as in tendinitis calcarea. Histologically, a calcium deposit rather than new bone would be formed. As yet, there is unfortunately no consensus on the definition and classification of heterotopic ossification.5
Martin et al regard myositis ossificans and heterotopic ossification as subsets of the process of ectopic bone formation: myositis ossificans being confined to muscle and heterotopic ossification as the ossification of capsular structures and joints.6 Although some authors have regarded them as separate processes,4 for the purpose of this chapter the term ectopic bone formation (EBF) will be used to encompass both. Also known as myositis ossificans circumscripta, myositis ossificans traumatica, extraosseous localized non-neoplastic bone, myo-osteosis, ossifying haematoma and traumatic ossifying myositis,3 it is a localized, self-limiting, reparative lesion that is composed of reactive hypercellular fibrous tissue and bone.1 A preceding traumatic incident is believed to be the initiating event in most cases. It is regarded by some as a rare, aberrant response to soft tissue trauma that passes through the same histological phases that are seen with fracture callus.7
Background/aetiology
The formation of bone in soft tissue requires inductive signalling pathways, inducible osteoprogenitor cells and a heterotopic environment conducive to osteogenesis.8 This triggers the transformation of mesenchymal cells into bone-forming cells.9 Differentiation of pluripotential mesenchymal cells into osteoblastic stem cells peaks at approximately 32 hours after surgery in mice.10 Thus, in this model, the crucial pathophysiological events occur in the immediate postoperative period, even though the EBF is not clinically or radiologically detectable for many weeks. Although it is known that this differentiation is induced by bone morphogenic protein,11 little is known about the molecular pathogenesis of this condition. Research into the genetic forms of this disease has revealed that there is overexpression of bone morphogenetic protein-4 and underexpression of multiple antagonists.12
Although the exact aetiology of EBF is unknown, there are a number of recognized associations or triggers. The first and most common association is the posttraumatic type with a single traumatic event or repetitive trauma being noted in 40–75% of the published cases.1,7 Thompson and Garcia have given a figure of 3% for the number of patients that develop EBF following simple elbow dislocations.13 Ilahi et al reported that the incidence rose to almost 50% in patients with fractures about the elbow (n = 41).14 After a direct blow to a muscle the incidence has been reported between 9% and 17%.15 A similar but atraumatic type has also been described. The proposed mechanisms for this type include non-documented trauma, repeated small mechanical injuries or ‘microtrauma’,3 and non-mechanical injuries as a result of ischaemia or inflammation.2,16 This type is usually confined to one muscle or muscle group. Factors that predispose to developing EBF after an injury to an elbow include a previous history of EBF, male gender, age greater than 60 years, Paget’s disease, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis (Forestier’s disease) and hypertrophic osteoarthrosis.12
Circumscribed heterotopic new bone formation without a history of trauma is sometimes regarded as a separate entity and is termed pseudomalignant EBF due to the diagnostic confusion between this benign lesion and other malignant conditions. The patient presents with a localized expanding soft tissue mass that exhibits peripheral calcification surrounding a radiolucent centre on plain radiographs and CT scans.17 The condition is benign and self-limiting and is indistinguishable from atraumatic EBF. A variant that is associated with long periods of immobility or a vegetative state has been described in paraplegics (the prevalence being reported as between 20% and 25%)18 and traumatic brain injury patients,19 and also as a rare complication in patients with tetanus, poliomyelitis and burns.6 Karapinar and Yagdi reported a case of a female with tetanus whose elbows became ankylosed in extension.20 Tompkins and Lachiewicz also described the development of disabling myositis ossificans of the posterior aspect of the elbow in a 66-year-old man as a sequela of tetanus.21
EBF following distal repair or reattachment of biceps brachialis is also well recognized. Of the two approaches to the repair the anterior repair alone has the advantage of a minimal risk of heterotopic bone formation, although it carries a greater chance of injury to the posterior interosseous nerve. Conversely, the two-incision technique markedly diminishes the risk of posterior interosseous nerve palsy, but is associated with a greater likelihood of EBF.22
Pathology
EBF is considered to be a pseudosarcomatous condition by virtue of its clinical and histological features,23 including reactive hypercellular fibrous tissue and bone. Although it is clearly a benign lesion, its clinical, radiological and histological appearance may sometimes mimic a malignant tumour.24 Histologically EBF cannot be distinguished from fracture callus.5
Immediately following the traumatic incident there is haemorrhage and fibrin deposition into the injury site. The centre of the ‘wound’ will contain variable amounts of necrotic material, blood clot and fibrin.7 Unlike other contusional injuries the wound displays an aberrant mesenchymal reaction directed towards callus formation. Under the microscope, the lesions exhibit a wide range of histological features with different amounts of immature fibroblastic cells, osteoid, cartilage, and young or mature bone accompanied by fibrous connective tissue. Comparing these appearances with those of pseudomalignant myositis ossificans, histologically there is microscopic evidence of zonation in which peripheral maturation is present with the central proliferating zone usually causing the diagnostic problems. This zonation is a characteristic of EBF and differentiates it from extraskeletal osteosarcoma.7 This differentiation was recognized as long ago as 1958 when Ackerman described how the bone is formed first at the periphery of the lesion in EBF but in sarcoma calcification extends centrifugally.2,4
Using electron microscopy, Povysil and Matejovsky demonstrated cells showing the morphological features of myofibroblasts and monocytic cells of the macrophage type. They considered that these previously unreported features together with the zonal pattern of the lesions indicated their reparative nature.25 EBF represents a polyclonal hyperplastic proliferative, regenerative process. This has been demonstrated by Leithner et al.24 The osteoinductor releasing cells are blood-borne monocytoid cells which enter the tissues by diapedesis and become histiocytes, macrophages, matrixclasts and osteoclasts; their precursors are derived from bone marrow at sites remote from the area of bone induction. The cell populations responding to the osteoinductor released by this mechanism develop into osteoblasts and osteocytes, and are the progeny of perivascular mesenchymal cells.26
Presentation, investigation and treatment options
Presentation
EBF occurs particularly in young and vigorous male adults participating in sports such as football.27 This male preponderance is consistent with the fact that males are more susceptible to injury in sports than are females.7 Geschickter and Maseritz reported on 25 patients with EBF. Fifteen gave a history of injury and 21 were males.28 However, Mirra stated that up to 50% of patients may not give a history of trauma when the patient understands the type of trauma necessary to induce the onset of EBF. With a detailed history and careful estimation of the age of the lesion, however, a history of trauma can be elicited in most patients.7
The presenting symptoms may develop over a number of days or weeks and can consist of one or more of the triad of local pain, a hard palpable mass in the muscle and a flexion contracture of the elbow.29 These symptoms have been noted to develop up to a year after the injury.30,31 Unexplained increase in pain, relative increase in spasticity or guarding of muscle should alert the examiner to the possibility of EBF regardless of the aetiology.18 However, the condition can be asymptomatic and may be diagnosed either incidentally2 or because of progressive loss of motion of the elbow.6
The clinical examination provides important diagnostic information. Usually, the earliest physical findings are those of a doughy mass present within a few hours of the injury.28 Increased joint stiffness, a limited range of motion, warmth, swelling and erythema are the principal clinical signs.5 The most common, and often the earliest, clinical sign is limited range of motion.18 The next most common sign is localized swelling.30 Because these clinical signs are not exclusive to EBF and are common in inflammatory conditions they cannot be regarded as pathognomic of EBF. The early inflammatory stage may mimic cellulitis, thrombophlebitis, osteomyelitis or a tumorous process.32
Sites
The most common sites for the development of ectopic bone around the elbow are medially and laterally where the bone is adjacent to or surrounding the collateral ligaments.33 Posterolateral is the most frequent site to ankylose and, consequently, the most common site about the elbow to require surgical resection.18 Lateral EBF in the vicinity of the radial collateral ligament occurs more commonly after traumatic EBF.33 Anterior ectopic bone is usually formed beneath the brachialis muscle and posteriorly it is usually deep to the triceps.18 When the ectopic bone surrounds the ulnar nerve, an entrapment neuropathy may ensue.34,35
Investigations
Laboratory investigations
The laboratory investigation of choice is the assessment of serum alkaline phosphatase (AP).18 Elevated levels occur after 3 weeks,18 and 4 weeks after injury AP levels may reach 3.5 times the normal value, with a peak concentration around the twelfth week. If the EBF is small, AP levels may remain unchanged.31 When the levels are elevated, this is a good parameter to assess, but only in the absence of fractures.5 Contrary to these findings in adults, AP levels are not elevated in children.19 Prostaglandin E2 (PGE2) excretion in 24-hour urine is felt to be a reliable bone marker not only for the early detection but also for determining the efficacy of treatment. A sudden increase in 24-hour urinary PGE2 excretion would be an indication to perform bone scintigraphy.6 The erythrocyte sedimentation rate (ESR) can also be elevated.36 Calcium and phosphorus levels are often elevated or may remain normal, making these unreliable markers.37
Three-phase bone scintigraphy
Three-phase bone scintigraphy is usually positive for EBF after 2–4 weeks18 and is the most sensitive imaging modality for the early detection of EBF. Repeated bone scans can be used to determine the optimal timing for surgical resection (see below), and to monitor postoperative recurrence.5
Ultrasonography
Ultrasonography detects EBF sooner than does conventional radiography.38 Thomas and Amstutz described the zone phenomenon that is evident on ultrasound scans and is specific for EBF.39 Ultrasound scanning is regarded by a number of authors as being the best investigation not only for the early detection, but also for the follow-up of EBF5,40 as well as for distinguishing the condition from extraosseous sarcomas.40
Radiography
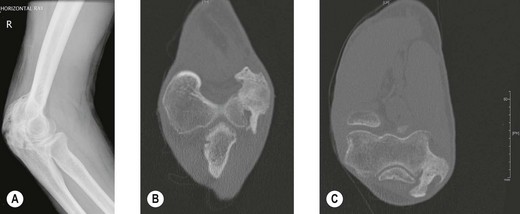
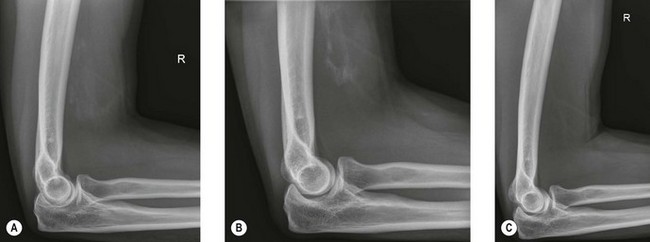
Radiography (Fig. 50.1A) and computed tomography (CT) (Fig. 50.1B,C) have low specificity in the early stages of EBF. As part of the surgical planning process, CT in combination with magnetic resonance imaging (MRI) are valuable to assess the spatial relationships with other anatomical structures such as blood vessels and peripheral nerves. Angiography is rarely used for the diagnosis of EBF, but may aid in delineating important vessels in cases of massive EBF.5 New bone is demonstrable on radiographs from 4 to 6 weeks after the injury (Fig. 50.2A). Geschichter and Maseritz showed that the first evidence of ossification was a small, dense shadow in the soft tissue some distance from the bone,28 although Gruca claims that bone can be seen as early as 3–4 weeks, reaching a maximum size from 10 weeks to 6 months41 (Fig. 50.2B). This has been corroborated by Chantraine and Minaire.42 Mirra recommended that to elucidate the early calcifications in EBF radiologically the kilovoltage must be kept within the range used for weak soft tissue densities.7
Magnetic resonance imaging
EBF typically presents as a soft tissue mass and MRI is commonly used to evaluate such swellings. de Smet et al scanned seven patients with EBF to determine if typical patterns were present.43 They concluded that typical MRI appearances of EBF do exist. A low signal intensity rim was a common finding. However, the patterns are not unique to EBF and resemble those that have been reported in other lesions. They warned that it is important to be aware of the spectrum of MRI findings of EBF when considering the differential diagnosis of a soft tissue mass.43 Kransdorf et al found that on T2-weighted spin-echo images, the lesions were relatively well defined and heterogeneous with intermediate to high signal intensity.44 When compared with the histology of the lesions, the latter corresponded to a central proliferating core of fibroblasts and myofibroblasts with a myxoid stroma resembling nodular fasciitis, rimmed by osteoblasts with bone production. Oedema surrounded lesions less than a few months old. T1-weighted images of early lesions were normal or showed evidence of a mass by displacement of fat planes. Mature lesions tended to be well defined with heterogeneous signal intensity, similar to that of fat, representing areas of fat situated between bone trabeculae within the lesion. Kransdorf et al noted that the varying appearance of EBF related to the histological changes that occur as the disorder progresses.44
The use of gadolinium is useful in distinguishing between EBF and malignant lesions. Cvitanic et al found that unenhanced sequences could be helpful in excluding malignancy, particularly when viewed serially.45 While the use of gadolinium can allow primary sarcoma to be excluded, it is not useful in the exclusion of early abscess formation or necrotic metastasis45 because the MRI features mimic those of an inflammatory mass or neoplasm. MRI is non-specific in the diagnosis of the early stages of EBF.46
Clinical Pearl 50.4
Biopsy
The diagnosis of EBF in the early stages is difficult. A needle aspirate of the lesion will determine if the mass is solid or contains pus. If it is solid and a biopsy is contemplated then this should be delayed because an early lesion of EBF is difficult to distinguish from a sarcoma. Close monitoring should be implemented with AP measurements, PGE2 excretion in 24-hour urine, bone scans, X-rays and CT scans (see above). Once a wedge or excisional biopsy has been performed of an ‘unripened’ mass it is very important to give as much clinical information to the pathologist as possible – history of trauma, behaviour of the lesion, rate of growth and the results of laboratory investigations. The lesion may recur within 2 weeks and the patient should be made aware of this. The growth of this reparative lesion should, however, cease after 7 weeks and is rarely larger that half the size of the original.7 Mirra recommends total excision of the remaining mass and the recurrence within 4 weeks to ensure the benign nature by the appearance of maturation and zonal patterns.7
Treatment options
EBF is difficult to treat once the process has been initiated, therefore it is important to begin prophylactic treatment as early as possible in susceptible patients.6 Some patients with minimal ectopic bone may require no specific treatment whereas others may require the full gamut of treatment including medication, physiotherapy, radiotherapy and surgery.
Natural history
The natural history of EBF when recorded radiologically is similar and predictable regardless of the aetiology. Only minor variations are seen between the different aetiologies and within similar patient populations.18 Most of the studies of the natural history of the condition are of EBF following total hip arthroplasty or in traumatic brain injury patients. From these studies some inferences can be made regarding ectopic bone formation about the elbow. The growth of the mass is usually complete by 7–8 weeks7 and the majority of ossification occurs by 3 months after the initial injury with radiological evolution for up to 6 months. In some patients with spinal cord injury, increased scintigraphic activity within ectopic bone may persist for some years.54,55 Occasional total regression has also been reported by Leithner et al24 and others56 (Fig. 50.2C).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree