3.2 Myofascial force transmission Endomysia constitute tubes for each myofiber. Note, however, that endomysial walls are shared between adjacent tubes (and myofibers). This causes a continuous honeycomb type of structure of muscular connective tissues until the fascicle borders are reached (see Chapter 1.1). The collections of fascicles are made up in a similar way, having the perimysium as borders delimiting fascicles, so that within a muscle a stroma of continuous tubes is present that is delimited by the epimysium, surrounding the whole muscle. Myofascial force transmission limited to the intramuscular domain is called intramuscular myofascial force transmission. This term is used also in the case of force transmission of a single myofiber or fascicle operating within its endomysial or perimysial tunnels (Ch. 8.4). In those cases, and that of a fully dissected muscle, reaction forces have to be exerted via tendons as these are then the only effective connections to the outside world (Huijing et al. 1998). If a myofascial load (reaction force from fascial structures) is exerted onto muscle (Huijing & Baan 2001), force is transmitted onto the intramuscular stroma via the epimysium. Therefore, such transmission is called epimuscular myofascial force transmission. Two pathways are available for such transmission: • Directly between two adjacent muscles (occurring exclusively within a muscle group: synergistic muscles). We call this specific case intermuscular myofascial force transmission. • Between a muscle and some extramuscular structures, such as the neurovascular tract (i.e., the collagen-reinforced structure in which blood vessels, lymphatics and nerves are embedded), intermuscular septa between muscle groups, interosseal membrane, periosteum, general (or deep) fascia, etc. We call this extramuscular myofascial force transmission to emphasize the role of extramuscular tissues. Forces exerted this way may play a role in stabilizing joints, or be exerted on bones and other extramuscular structures, but may also be exerted at other muscles. All of the tissues discussed above (with the exception of the aponeuroses and tendons) are part of a continuous fascial system. By itself, the fact that they are connected will not warrant force transmission; if the connections are very compliant (i.e., not stiff), force will only be transmitted after very high length changes that will stiffen connections. However, experiments indicate that also after more moderate length changes sizable fractions of muscular force may be transmitted. This means that fascial structures that are not dense depositions of collagen fibers may transmit some muscular force, and therefore it has been argued that the term “loose connective tissue” for such structures is inadequate (Huijing & Langevin 2009) and the term “areolar” is preferred for such tissues. Proximo-distal force differences Due to additional myofascial loading, forces exerted at the origin and insertion of muscle are not equal (Huijing & Baan 2001). The net myofascial load (i.e., the vector sum of all such loads, involving size and direction) will keep sarcomeres at one end of myofibers within muscle longer than at other locations within the same myofibers, i.e., different active forces exerted locally (Fig. 3.2.1A). A force equal to the additional load is integrated into the force exerted at the opposite end of the muscle. Active force of several muscles may appear also at the insertion of another muscle, as long as myofascial connections to the extratendinous tissues are intact (Rijkelijkhuizen et al. 2005, 2009). Fig. 3.2.1 • Myofascial force transmission and some of its consequences. If the point of application of myofascial loads on myofibers and their size and direction were identical, sarcomere length distributions would be limited to serial ones. However, a simple test illustrates that the situation is more complex. If one suspends equal masses from the tendon of a (horizontal) muscle, the muscle is pulled down exposing the extramuscular neurovascular tract (Plate 3.2.1A), but this tract is pulled down more at the distal tendon than at the proximal tendon. This indicates that the tract is stiffer at the proximal side of the muscle. As a consequence, myofibers located proximally within muscle will, on average, be longer than more distal ones. Therefore, parallel distributions of sarcomere lengths will be present as well. The nature of both types of distributions will vary with the specific conditions of myofascial loading. It is hard to measure such distributions, but finite element modeling (Ch. 8.5) allows the study of such principles even in quite complex conditions of loading. Dissection experiments (Maas et al. 2005) indicate that intermuscular transmission plays a role, but extramuscular myofascial force transmission is the more important mechanism for muscular interaction. Intermuscular mechanical effects are present through two coupled events of extramuscular myofascial force transmission (muscle to extramuscular tissues to another muscle). Myofascial interaction was shown for synergistic muscles involving both intermuscular and extramuscular transmission (Huijing & Baan 2001; Huijing 2003). During experiments this is apparent as follows: After the agonistic muscle is lengthened at its distal tendon while its synergistic muscle is kept at constant length (Plate 3.2.1B), distal force of the isometric synergistic muscle is decreased (compared to the unconnected case) with increasing lengths of its adjacent muscle. The lengthened muscle creates, or enhances, a distally directed myofascial load on the isometric synergistic muscle. At its proximal tendon, this load is integrated into force exerted by the muscle (as in Plate 3.2.1A). In contrast, distally exerted force is decreased because of such loading conditions. Myofascial force transmission between antagonistic muscles is (by definition) only possible via extramuscular mechanisms, as such muscles are separated by compartment walls. Stiff connections are made between compartments by neurovascular tracts, but also other extramuscular fascial structures are expected to play a role. The effects and explanation of myofascial interaction between two muscle groups located at opposite sides of an intermuscular septum or interosseal membrane (Plate 3.2.1B) are similar to those described for synergistic muscles (Huijing 2007; Huijing et al. 2007; Meijer et al. 2007, Rijkelijkhuizen et al. 2007). In a series of experiments in rats, we have shown that such mechanisms and effects are active between all muscles of the lower leg (for an overview of results, see Rijkelijkhuizen et al. 2009). So, even antagonistic muscles located at opposite sides of the leg interact (e.g., m. tibialis anterior and triceps surae muscles, see Ch. 5.8 for similar physiological results in mice), and cannot be considered as fully independent entities. It should be realized that this means that part of the force exerted by active sarcomeres within a muscle may be exerted at the tendon of its antagonistic muscle. In fact, the proximal sarcomeres of myofibers are in series not only with more distal sarcomeres of the same myofiber, but via myofascial loading also with distal sarcomeres (and their adjacent endomysia) in the lengthened antagonistic muscle(s). It is evident that the classical concept of antagonistic muscles (as having opposite mechanical effects) and the nomenclature used to describe them is due for a fundamental update… that, however, is beyond the scope of the present chapter.
An introduction
Intramuscular substrates of myofascial force transmission
Epimuscular myofascial force transmission and its substrate
Effects of epimuscular myofascial force transmission
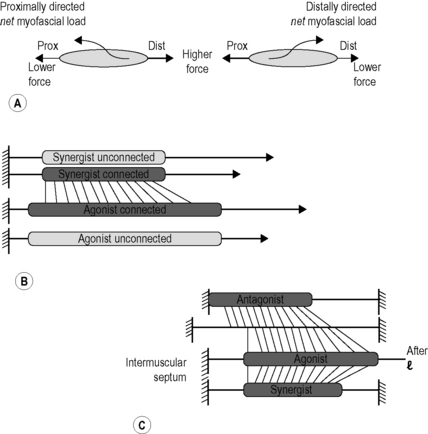
(A) Proximo-distal force differences. The net myofascial load on the muscle is integrated into either the proximal force (left panel) or the distal force (right panel) depending on the loading direction. (B) Comparison of distal forces of unconnected and myofascially connected muscles after distal lengthening. As the agonistic muscle is lengthened the force in the synergistic muscle drops. (C) Connections and myofascial loading of antagonistic muscles across the intermuscular septum. In these conditions part of the antagonistic muscle force will be exerted at the distal tendon (right) of the agonistic muscle.
Distributions of sarcomere lengths within muscle and its myofibers
Myofascial interaction between muscles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine






