1.3 Musculoskeletal Chronic Pelvic Pain
 Introduction
Introduction
A wide range of definitions of chronic pelvic pain (CPP) have been given in the literature. It has been defined as any pain lasting longer than 3 months, located anywhere from the umbilicus to the genital region [Zondervan and Barlow 2000]. Other authors have differentiated CPP from dyspareunia, dysmenorrhea, and endometriosis [Mathias et al. 1996]. In a general population study, it was estimated that 9.2 million women in the USA had CPP [Mathias et al. 1996]. In the United Kingdom, the prevalence of CPP in women aged 18–50 was found to be 24% [Zondervan et al. 1998]. The frequency of this disability was similar to individuals diagnosed with low back pain [Zondervan et al. 2001a]. Annual direct costs for treatment of pelvic pain in the USA are estimated to be $881.5 million [Mathias et al. 1996]. In the United Kingdom, CPP with negative laparoscopy findings was estimated as costing cost £182 million [Zondervan et al. 1998]. In a clinical population study in the USA for women seeking treatment in a general gynecological practice, 39% reported chronic pelvic pain [Jamieson and Steege 1996]. The prevalence of this impairment in the USA and United Kingdom—as well as the estimated financial impact—could be much greater, given the fact that many women are reluctant to discuss pelvic pain symptoms due to cultural and psychological inhibitions [Jamieson and Steege 1997, Zondervan et al. 2001a]. A clear definition of the symptoms related to CPP is difficult to ascertain from the literature. In a recent review of the medical literature, it was found that the term CPP is poorly defined, making it difficult to carry out a comparative analysis of the treatment of the condition [Zondervan et al. 2001b]. The degree to which musculoskeletal dysfunction contributes to the prevalence of CPP has not been studied. Dyspeunia, low back pain, and lower abdominal discomfort have been reported to coexist in individuals with CPP [Porpora et al. 1999]. For the purposes of this discussion, musculoskeletal CPP will therefore be defined as pain located in the region of the abdomen, below the umbilicus, and in the region of the pelvis. musculoskeletal impairments related to dyspareunia will also be discussed.
Several authors have established the treatment of musculoskeletal impairments related to CPP [Browning 1987, 1989, 1990, Baker 1993, AlSalilli and Vilos 1995, Condon et al. 1995, Ekberg et al. 1996, Hall and Brody 1998, Steege et al. 1998, Wergeland and Strand 1998, Carter 1999, Ehlert et al. 1999, Porpora et al. 1999, Treloar et al. 1999, Markwell 2001, Mens et al. 2002]. A relationship between back pain, dyspareunia, and CPP was reported by Gurel and Atar [1999]. This study, along with the large numbers of clinical reports noted above, provides evidence of the need to look at impairments of musculoskeletal structures as a cause for CPP. Yet many primary-care clinicians in health care today do not understand the mechanisms for musculoskeletal pelvic pain. Musculoskeletal CPP therefore remains unrecognized and untreated. A lack of response to standard medical care for pain in the region of the pelvis or abdomen will cause the primary-care clinician to consider a psychological diagnosis and fail to investigate alternatives. Pain in the region of the pelvis and abdomen must be medically screened for gynecological, gastrointestinal, and urological origins [Goodman and Boissonnault 1998, Stones and Mountfield 2000, Zondervan et al. 2001a]. If screening proves negative, then a musculoskeletal examination is warranted. Misdiagnosis can commonly cause patients to seek services from multiple physicians and to undergo pharmacological and surgical interventions [Walker et al. 1993, Baskin and Tanagho 1991, Chen and Soong 1997, Lindheim 1999, Stones and Mountfield 2000] before being referred to a physical therapist.
A physical therapist is specifically trained to diagnose movement impairments. Movement impairments are abnormalities in key components of motion resulting in pain and functional impairments [Sahrmann 2002]. In the case of musculoskeletal CPP, the cause of cramping and aching in the lower abdominal and pelvic region may be diagnosed by a physical therapist as a rotational movement impairment of the lower thoracic spine [Grant 2002]. Rotational movement impairments would present with an excessive amount of motion of the thoracic spine in the direction of rotation during functional activities. Thus, the increased motion will place a stress on the neural structures originating in the thoracic region. This compromise of the neural tissue will produce a referred pain into the abdomen [Bannister et al. 1995, Peleg et al. 1997]. Typically, as in this example, cramping and aching felt in the lower abdominal and pelvic region would be treated with a medicine-based approach for a suspected diagnosis of endometriosis [Laufer et al. 1997, Walter et al. 2001]. Definitive diagnosis for this disease is achieved by observing endometrial tissue during laparoscopy of the abdomen [Walter et al. 2001]. In an effort to avoid this invasive procedure, a physician would often prescribe multiple medications related to hormone regulation [Damewood 1993].
Physical therapy intervention for CPP patients is determined by examination of the motions of the trunk and extremities with a correlation of changes in the patient’s symptoms with correction of the observed movement impairments. As with any musculoskeletal dysfunction, the first step for the clinician is to identify the types of tissue alteration that have occurred with repeated movements and sustained postures [Grant 2002]. Tissues that potentially could be involved with CPP are nerves, muscles, ligaments, and cartilaginous structures of the spine, pelvis, and hips [Baker 1993]. Several trunk and hip muscles are synergists of the pelvic floor musculature (PFM) [Bannister et al. 1995, B⊘ and Stein 1994]. Muscular dysfunction in the pelvic floor muscles has been related to pain in the lower abdominal and pelvic region [Hunter and Zihlman 1970, Al-Salilli and Vilos 1995, Jamieson and Steege 1996, Goodman and Boissonnault 1998, Wergeland and Strand 1998, Ehlert et al. 1999, Mayer et al. 1999, Treloar et al. 1999]. Pelvic floor muscle examination by the physical therapist is therefore critical to define the scope of the symptoms. The coexistence of urological symptoms such as stress incontinence or dyspareunia (a muscle performance impairment) or urgency (a neural performance impairment) is common in patients with CPP [Zondervan et al. 2001b, Weiss 2001]. Throughout the discussion of general muscle function in this section, specific applications to the PFM will be discussed. Differentiation of specific muscle impairments is imperative for accurate prescription of treatment.
Confusion in determining the cause of CPP symptoms is possible due to the common neural pathways that are shared by pelvic organs and musculoskeletal structures of the trunk and lower extremities [Steege et al. 1998, Wald 2001]. In addition, the large autonomic nervous supply in the region of the pelvis causes the perception of pain to be more diffuse than in the extremities [Abbott 1990, Bannister et al. 1995, Wald 2001]. Clinical observation reveals a slower rate of improvement with the correction of movement impairments that are inappropriately stressing neural structures in the pelvic region than with treatment of primary musculoskeletal pain [Hoppenfeld 1976]. Comorbidity of mechanical and nonmechanical pain can occur, so that it is paramount to differentiate mechanical pain [Walker EA et al. 1993]. A primary indication for a physical therapy consultation is if soft-tissue differentiation, pain reproduction with muscular or ligamentous palpation, and pain with contraction are positive [Mueller and Maluf 2002, Tipler 2002]. Secondary indicators include a correlation of symptoms to an activity or a sustained posture that would affect the musculoskeletal structures of the trunk, pelvis, and lower extremities. The correction of mechanical pain problems can aid a surgeon in the decision to remove a dysfunctional organ in women with concurrent nonmechanical pelvic pain.
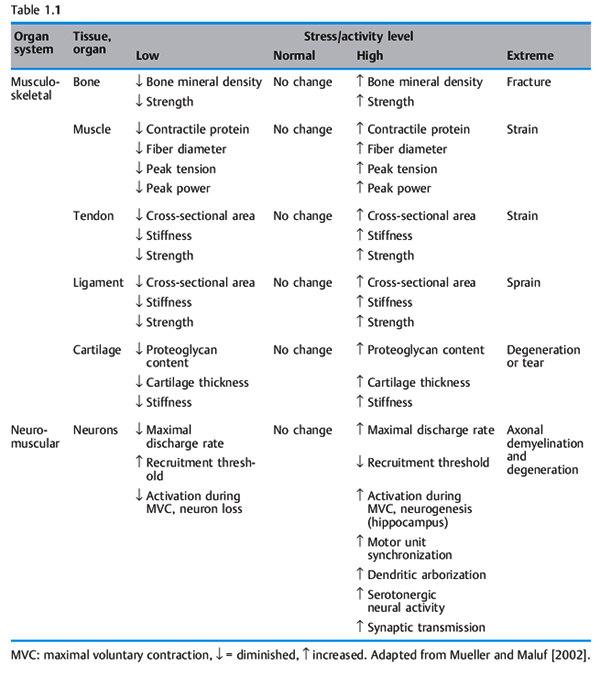
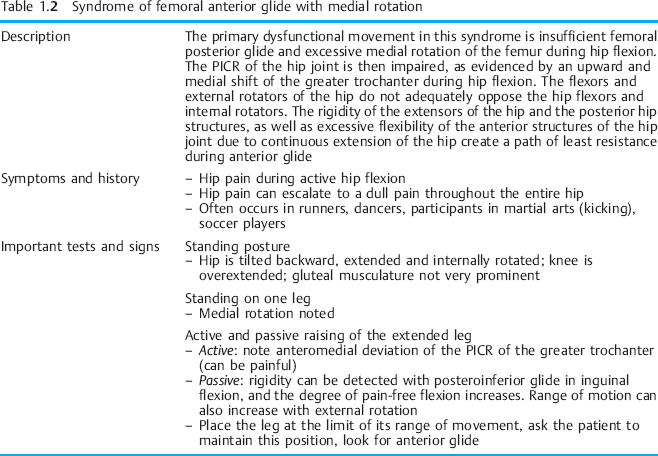
The physical stress theory proposed by Mueller and Maluf [2002] describes the relationship between mechanical stress on biological tissue (bone, nerve, muscle, connective tissue) and musculoskeletal pain syndromes. Physical stress is defined as the force that is applied to any area of biological tissue [Tipler 2002]. Table 1.1 gives a description of the tissue responses to stress. According to the physical stress theory, the application of an inappropriate amount of physical stress, such as movement, can cause a spectrum of responses [Mueller and Maluf 2002]. These responses include atrophy of a tissue due to lack of physical stress, hypertrophy due to an appropriate increase in stress, and injury due to excessive physical stress. Chronic tissue injury resulting in pain lasting longer than 8 weeks causes a lowered threshold of tolerance to physical stress [Mueller and Maluf 2002]. According to Sahrmann, imbalances of the movement system cause microtrauma to musculoskeletal soft tissues [Sahrmann 2002]. This micro-trauma will progress to macrotrauma, and tissue destruction occurs, resulting in musculoskeletal pain syndromes. Mueller and Maluf [2002] propose that a clinician should ask the following questions when evaluating a chronic pain syndrome: firstly, what factors appear to be contributing to excessive stress on the injured tissues? Secondly, how can these contributing factors be modified to reduce stress on the tissue and allow the tissue to heal? Sahrmann [2002] proposes that correction of muscle imbalances, faulty movement patterns, and sustained postures will reduce the mechanical stress on biological tissues and allow injured tissues to heal. Sahrmann continues that the painful tissues are considered the source of an individual’s symptoms. The cause of the symptom must be determined from the underlying movement impairments. With regard to CPP, there is a need to focus treatment not just on the specific painful structures but more importantly on the cause or causes. For example, consider an iliopsoas muscle strain that results in abdominal and groin pain; a clinician will not be successful in long term relief of symptoms if he or she fails to determine the cause of the symptoms—specifically, the underlying movement impairment. Correction of the accessory movement impairment of femoral anterior glide with medial rotation [Sahrmann 2002] would remove excessive physical stress on the iliopsoas muscle that has become chronically strained due to repeated posturing into a lengthened position. The path of the instantaneous center of rotation (PICR) of the hip joint is restored. Thus, the cause of the tissue irritation, a repeated physical stress on the structures of the hip joint, is removed and the complaint of abdominal and anterior groin pain is diminished. Treatment would include:
- Education of the patient in self-management through body mechanics and motor performance during functional activities.
- Static positioning.
- Therapeutic exercise with an emphasis on correction of the specific impairments affecting movement [Sahrmann 2002].
This approach will remove excessive physical stress due to faulty movement and apply appropriate physical stress, thereby allowing the individual to move to a higher threshold for tolerance to activity.
In the case of CPP, it is difficult to identify the exact structure or structures involved as the source of the symptoms. For example, current tools that are available to clinicians for examination of the structures of the sacroiliac joint (SIJ) are not considered reliable [Sahrmann 2002, Perkins et al. 1998, Van der Wurff et al. 2000]. Pain in the region of the sacrum could be the result of ligamentous laxity, neural tissue irritation referred from the lumbar spine, or improper motor performance of the hip and trunk musculature [Hislop 1975, Prather 2000, Van der Wurff et al. 2000]. According to the principles of the physical stress theory, correction of the factors affecting tissue adaptations within the region of the SIJ would reduce the physical stress on the ligamentous structures of the SIJ and allow tissue healing to occur [Mueller and Maluf 2002]. Successful treatment of CPP that is a result of pain in the sacral region can focus on reducing stresses from above and below the SIJ without definitive identification of the source of the pain [Spitznagle and Van Dillen 2001].
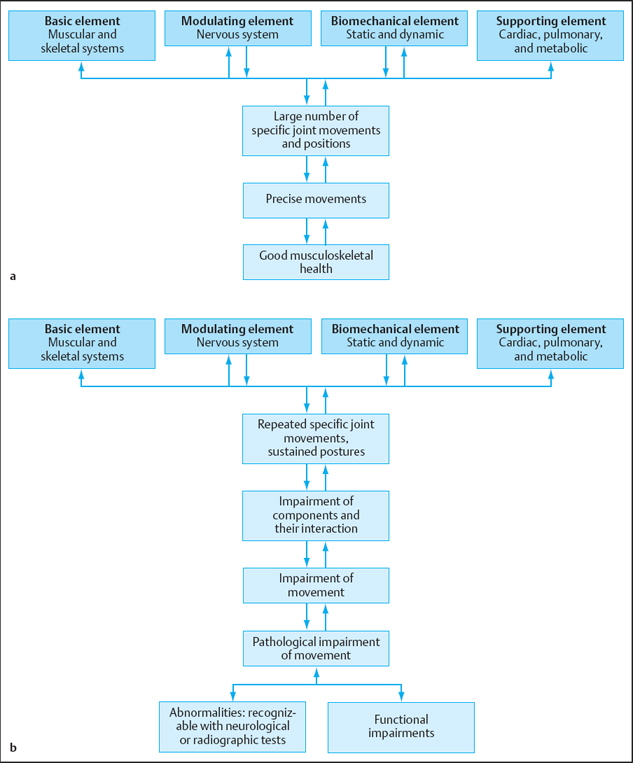
A kinesiological model was proposed by Hislop [1975] to describe the biological systems that contribute to the movement system; a model is shown in Fig. 1.22. The components of the model are:
- The base elements of the muscular and skeletal systems.
- Modular elements of the nervous system.
- Biomechanical elements including statics and dynamics.
- Support elements of the cardiac, pulmonary, and metabolic systems.
It is beyond the scope of this section to describe the relationship of the support elements to CPP. On the basis of Hislop’s model, Sahrmann [2002] proposed a kinesiopathological model to explain the way in which movement dysfunction develops (Fig. 1.22b). A change in one of the components of the model will lead to a cascade of imbalances which, if left unchanged, will result in musculoskeletal pain syndromes. Each component of the kinesiopathological model must be considered when diagnosing and prescribing treatment for a musculoskeletal pain syndrome. The present section includes: firstly, a description of common movement diagnoses for musculoskeletal CPP; secondly, a description of the movement examination for musculoskeletal CPP; and thirdly, a description of specific components elements of the kinesiopathological model as they relate to the examination of movement impairments that cause musculoskeletal CPP.
 Movement Diagnosis in Chronic Pelvic Pain
Movement Diagnosis in Chronic Pelvic Pain
The organization of impairments into a diagnostic category allows clinicians to systematically approach pain syndromes [Sahrmann 2002]. Musculoskeletal CPP can present with impairments from several joints, nerves, and soft-tissue structures. Pain can be referred from the spine, pelvic, and hip joints. There can be fascial restrictions due to postoperative scar formation or an underlying connective-tissue impairment. (It is beyond the scope of this section to discuss impairments related to scar formation.) Muscle impairments, including stiffness, weakness, and impaired motor control can be present. Neurological symptoms, numbness, or itching can be present without neurological signs. The overall rationale explaining why these impairments create pain syndromes needs to be determined. The course of treatment is based on this rationale. The movement system—a physiological system that produces motion of the whole body or of its component parts [Dirckx 1997]—is the basis for physical therapy practice [Sahrmann 2002]. It is therefore logical to diagnose a musculoskeletal pain problem with a movement diagnosis. Movement impairments can be broken down into physiological motions and accessory motions. The common movement impairment diagnoses seen in musculoskeletal CPP include the following:
- Hip impairments
- – Femoral anterior glide with medial rotation
- – Femoral adduction with medial rotation
- – Hip extension with knee extension
- – Femoral anterior glide with medial rotation
- Trunk impairments
- – Thoracic rotation
- – Thoracic rotation/flexion
- – Lumbar rotation
- Rotation/extension
- Rotation/flexion
- Rotation/extension
- – Thoracic rotation
- Pelvic impairments
- – Hypermobility of the SIJ
- Anterior iliac torsion
- Posterior iliac torsion
- Sacral rotation
- Anterior iliac torsion
- – Hypermobility of the pubic symphysis Superior/inferior glide
- – Hypermobility of the SIJ

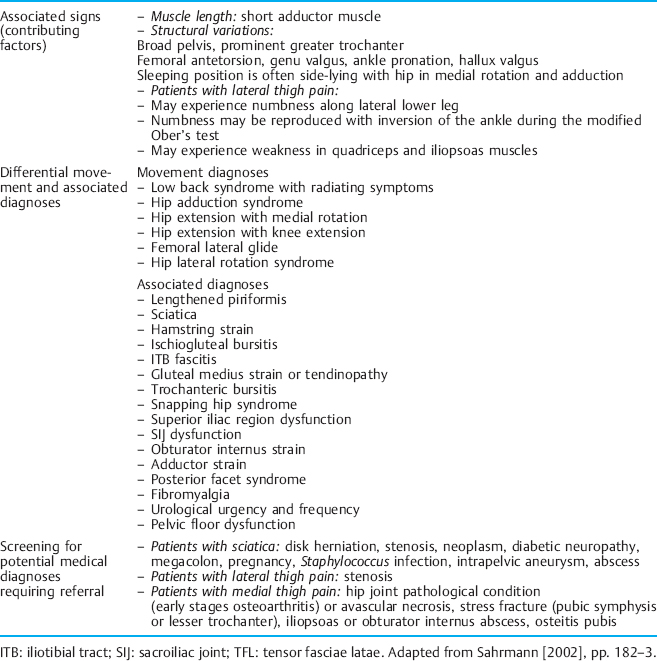
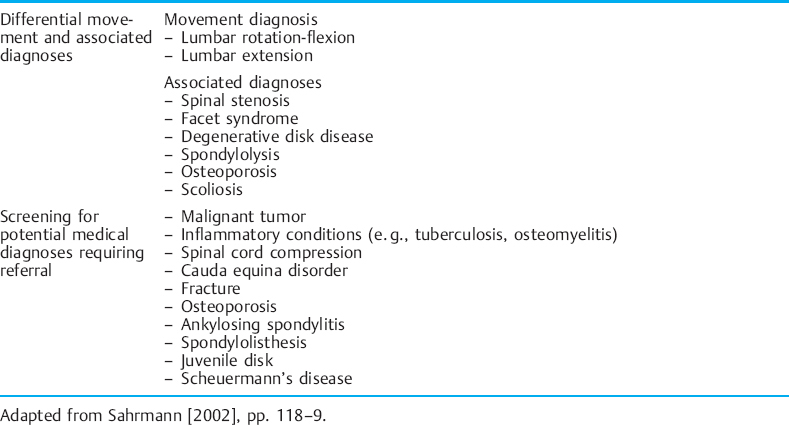
Tables 1.2–1.6 provide descriptions of some of these syndromes. The tables have been modified to include CPP symptoms. Examples of the way in which the movement diagnoses are applied to CPP are given throughout the discussion on the components of the kinesiopathological model. It should be noted that the treatment should focus on the cause of the dysfunction instead of the source. For example, in the case of a soft-tissue dysfunction such as tension myalgia of the PFM, treatment without regard to the whole movement system will greatly impair the treatment outcome.
 Movement Examination
Movement Examination
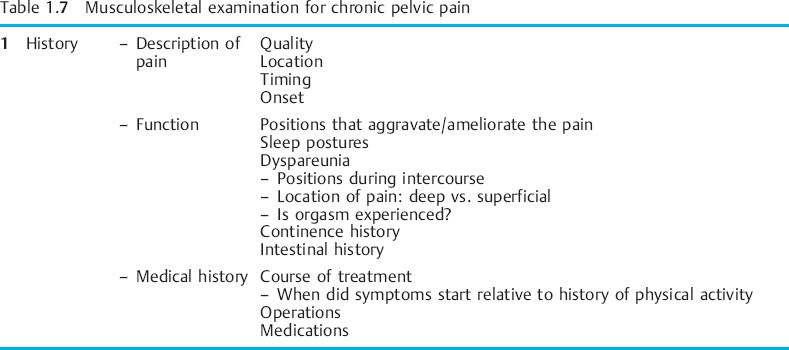
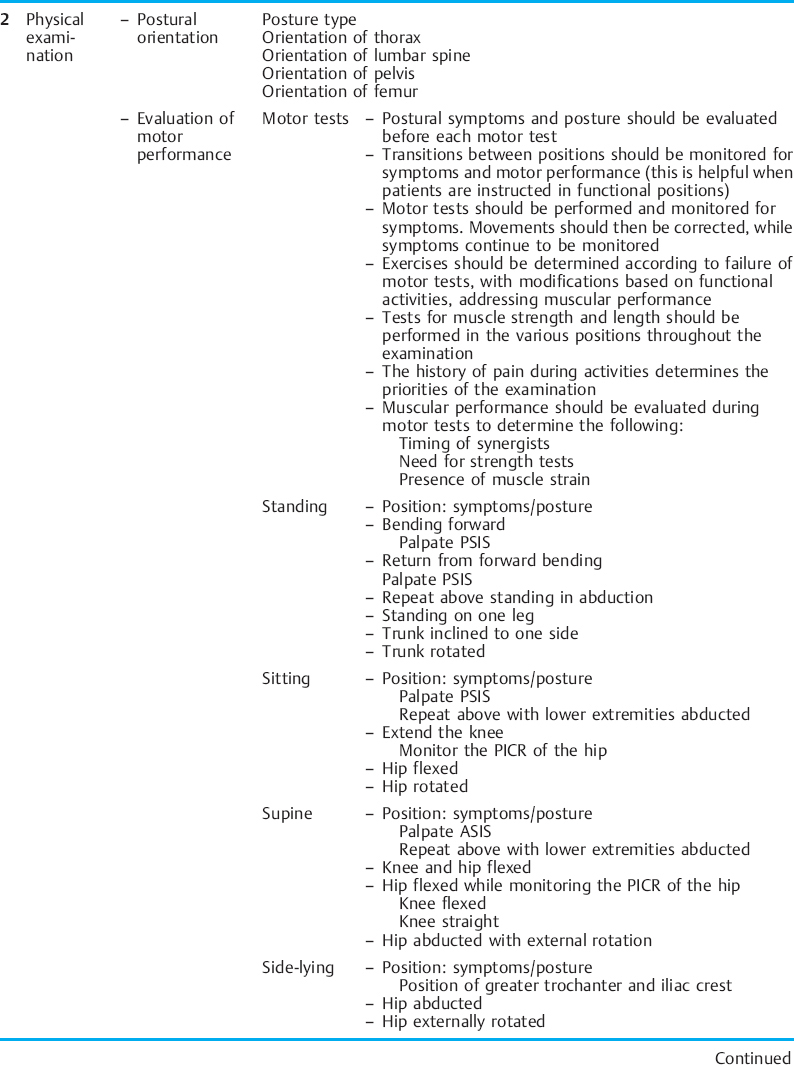
The movement examination as proposed by Sahrmann [2002] can easily be applied to the musculoskeletal CPP population. This examination is based on sampling movement in a variety of positions: standing, sitting, supine, side lying, prone, and quadruped. A movement examination includes tests that sample components of the kinesiopathological model. The components of a movement examination with emphasis on the structures that relate to CPP are shown in Table 1.7.
 Elements of the Kinesiopathological Model
Elements of the Kinesiopathological Model
 Base Elements
Base Elements
Postural Alignment
The association of posture with pain syndromes was initially proposed by Kendall et al. [1993]. Faulty postures in sitting and standing have been reported to cause deterioration in pelvic pain symptoms [Treloar et al. 1999]. However, several attempts to study the relationship of static posture to pain syndromes have failed [Hansson et al. 1985, Walker ML et al. 1987, Kendall et al. 1993, Christie et al. 1995, Youdas et al. 1996, 2000]. Sahrmann [2002] proposes that alignment should be considered a baseline for the initiation of movement. If movement does not appear faulty or does not reproduce the pain syndrome, the postural impairment component of the exam is not weighted strongly for diagnosis. In the treatment of musculoskeletal CPP, there are several points to consider in regards to postural impairments. Foremost, the difference between an acquired and a structural impairment should be understood. An acquired postural impairment is considered flexible and will not be present when the posture is modified. On the other hand, a structural impairment will not change with positional changes. Clinically, when examining for acquired postural impairments in musculoskeletal CPP, apparent leg length and sway alignment impairments are common.
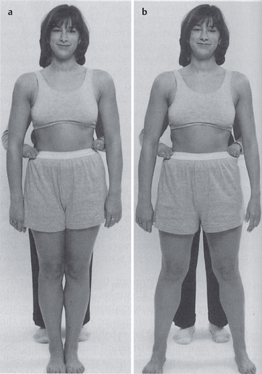
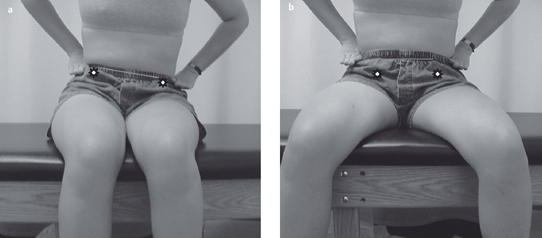
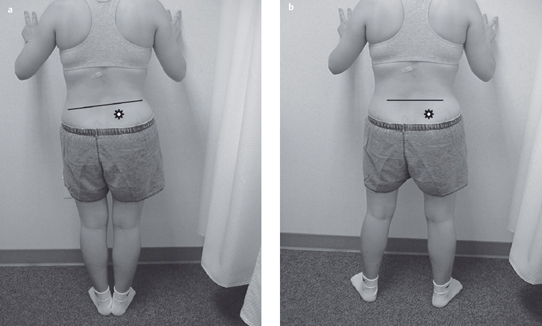
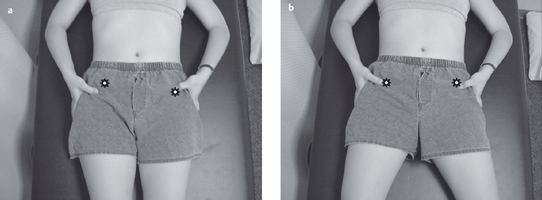
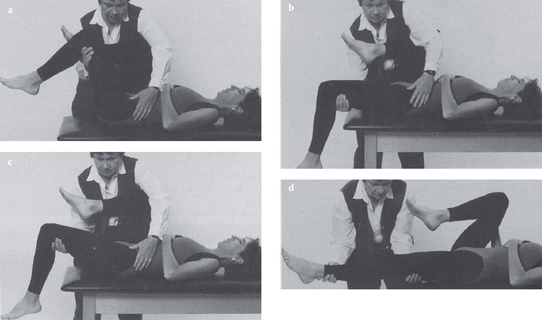
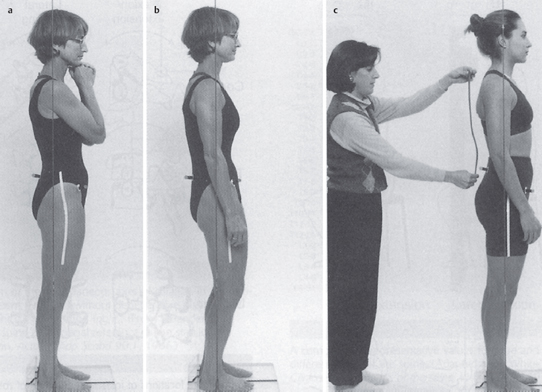
Acquired postural impairments. In an apparent leg length postural impairment [Sahrmann 2002], the apparent high leg, assessed in standing with the hands on the iliac crests, will levelwhen the lower extremities are placed in abduction. In this case, the asymmetry noted in standing is not a structural impairment, but is instead a flexible impairment that will respond to treatment. In standing, the high iliac crest has a relatively less stiff hip abductor and/or has an increase in length, while the low iliac crest has the relatively more stiff hip abductor and/or has a decrease in length. Figure 1.23 illustrates an apparent leg length discrepancy examination. In musculoskeletal CPP, all of the bony landmarks of the pelvis should be tested in the same fashion. The examination of pelvic landmarks, including the posterior superior iliac spine (PSIS), the anterior superior iliac spine (ASIS), and the iliac crest height in various hip positions will allow the physical therapist to determine the presence of a structural or acquired impairment of pelvic alignment. Figures 1.24–1.26 show the pelvic landmark examination in standing, sitting, and supine positions.
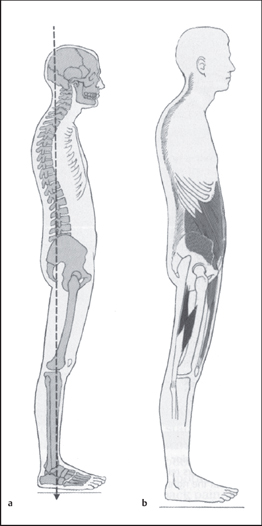
A typical pelvic pain posture (TPPP) of lumbar lordosis or kyphosis/lordosis as described by Kendall et al. [1993] has been reported. According to Baker et al. [1992], the TPPP includes an anterior pelvic tilt, lumbar lordosis, hyperextension of the knees, and a line of gravity displaced anteriorly to the lower extremities and pelvis. Baker et al. go on to describe common muscle imbalances observed in association with the TPPP. Iliopsoas shortness has been found to be one of the common impairments [Baker et al. 1992, Baker 1993]. It can logically be argued that anterior pelvic tilt would occur with a pelvic postural alignment of hip joint flexion, due to shortness of this one-joint hip muscle. Clinically, a long iliopsoas, as seen in the swayed alignment impairment [Kendall et al. 1993] with a short tensor fascia lata iliotibial band complex, is another common posture observed in CPP [Sahrmann 2002]. Figure 1.27 illustrates the swayed alignment fault. Accurate testing of the length of all the hip flexors requires diligence in positioning and test performance in order to differentiate the contributions of the iliopsoas, rectus femoris, and tensor fascia lata. Figure 1.28 illustrates the hip flexor length test [Sahrmann 2002].
Clinically, it has been observed that a sway alignment impairment is common in the middle-aged to younger female population instead of a lordotic/kyphotic alignment impairment. In a posturals way alignment impairment, the line of gravity falls behind the pelvis and lower extremities. This posturing would better explain the knee hyperextension seen in the TPPP. The swayed alignment can be difficult to visualize. Identification of this change in the line of gravity requires aligning the observation eye at the level of the greater trochanter, followed by visual appraisal ofthe shoulders in relation to this landmark. The resulting spinal alignment from a swayed thoracic spine to a flat lumbar spine can appear dramatic; to a cursory glance, it appears as lumbar extension. Accurate depiction of the lumbar alignment may require the aid of a flexible ruler. The increased prevalence of a sway postural impairment may be due to a change in muscle length in response to prolonged sitting, as has been induced by the avid use of computers and a more sedentary lifestyle. The change in the center of mass (COM) that occurs with pregnancy may also contribute to the prevalence of this impairment. The discrepancy found between the findings reported by Baker et al. [1992] and observations in other clinical settings should encourage further investigation of this condition.
Structural impairments. Due to two known structural impairments of the female pelvis— low ASIS (Fig. 1.29) and femoral antetorsion (Fig. 1.30) [Sahrmann 2002, Magee 1997]— other postural impairments besides the TPPP should be considered when conducting an examination of patients with CPP. If structural impairments are missed, a cascade of erroneous assumptions may occur regarding muscle length and strength, thereby leading to false postural impairments.
Low anterior superior iliac spine (ASIS).The first common female pelvic structural fault to consider in the examination of musculoskeletal CPP is a low ASIS (Fig. 1.29). Consideration of this structural fault was not reported in the study that described the TPPP. Women are more likely then men to have a low ASIS. The female pelvis has a wider flare to the iliac crests [Bannister et al. 1995]. It is critical to conduct an examination of alignment that combines information regarding lumbar spine alignment, hip joint alignment, and pelvic alignment to determine the presence of this structural fault. A low ASIS would give the initial impression that the pelvis is in an anterior tilt, due to the large difference in the height of the PSIS and the ASIS. Kendall et al. [1993] reported that the neutral pelvis should have up to a 5° difference between the ASIS and the PSIS. In the case of a low ASIS, the angle between the ASIS and the PSIS is far greater than 5°, yet the hip joint angle is neutral to extended and the lumbar spine appears flat. Correction of a postural impairment that has erroneously been determined to be an anterior pelvic tilt would result in a worsening of the alignment of the spine and hip joint angle (Fig. 1.29).
Femoral antetorsion. The second structural impairment to consider when examining patients with CPP is femoral antetorsion (Fig. 1.31).The average amount of hip medial rotation range of motion (ROM) is greater in females than males [Sahrmann 2002]. Sahrmann suggests that this occurs not only because the female pelvis is wider than the male pelvis, necessitating adduction and medial rotation to get the foot to the ground, but also that the structural alignment impairment of femoral antetorsion is more commonly found in females than in males. Gelberman et al. [1987] concluded that an increased likelihood for femoral antetorsion exists if hip medial rotation ROM is greater than the lateral rotation range of motion in both sitting and prone positions. Figure 1.30 shows an example of antetorsion. The Craig test (Fig. 1.32), as described by Hoppenfeld [1976], is carried out along with the above-mentioned ROM tests to determine the presence of this structural impairment. In the case of antetorsion, a neutral position of the hip joint, the greatest congruence of the femoral head with the acetabulum will cause the lower leg to posture in medial rotation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree