After reading this chapter the student or therapist will be able to: 1. Identify the various types of neurovascular disease. 2. Identify the atypical patterns of movement in clients with residual hemiplegia. 3. Identify significant primary and secondary body system problems (impairments) that interfere with functional movement patterns and limit ability to participate. 4. Describe a reeducation intervention strategy for improving functional movement in clients who have had a stroke. The treatment of hemiplegia from vascular insult is controversial. Various treatment methods have been devised and advocated. Recent scientific theories have changed the focus of treatment from one of inhibition of abnormal tone and facilitation of normal movement to reeducation of control and weakness, and functional retraining. In this chapter, pathological conditions, body system problems (impairment), functional limitations, and intervention strategies for clients with hemiplegia from stroke are reviewed. Although hemiplegia from neurovascular pathological conditions is the focus of the chapter, therapists can use this information and apply it to adults with hemiplegia caused by other central nervous system (CNS) pathological conditions, such as tumor (see Chapter 25), trauma (see Chapter 24), multiple sclerosis (see Chapter 19), and demyelinating diseases (see Chapter 17). Movement components and their relationship to functional performance are used as the basis for selection of therapy techniques and training. In the United States, stroke is the third ranking cause of death—more than 137,000 people die each year—and is the leading cause of adult disability.1 The National Stroke Association estimates that 795,000 new or recurrent strokes occur each year. The incidence of stroke rises rapidly with increasing age: two thirds of all strokes occur in people older than the age of 65 years; and after the age of 55 years, the risk of stroke doubles every 10 years. With the over-50-years age group growing rapidly, more people than ever are at risk. In the United States, the incidence of stroke is greater in men than in women, and it is twice as high in blacks as in whites. Cerebral infarction (thrombosis or embolism) is the most common form of stroke, accounting for 70% of all strokes. Hemorrhages account for another 20%, and 10% remain unspecified. Stroke is the largest single cause of neurological disability. Approximately 4 million Americans are dealing with impairments and disabilities from a stroke. Of these, 31% require assistance, 20% need help walking, 16% are in long-term care facilities, and 71% are vocationally impaired after 7 years.1 One study reported that 12% of subjects have complete functional arm recovery and 38% have some dexterity 6 months after stroke. In addition, loss of leg movement in the first week after stroke and no arm movement at 4 weeks are associated with poor outcomes at 6 months.2 The three most commonly recognized risk factors for cerebrovascular disease are hypertension, diabetes mellitus, and heart disease. The most important of these factors is hypertension.3 Because high blood pressure is the greatest risk factor for stroke, human characteristics and behaviors that increase blood pressure, including increased high serum cholesterol levels, obesity, diabetes mellitus, heavy alcohol consumption, cocaine use, and cigarette smoking, increase the risk of stroke. Ostfeld4 noted that mortality rates for stroke declined, slowly at first (from 1900 to 1950) and then more quickly (from 1950 to 1970), with a sharp drop noted around 1974. Experts have speculated that the greater use of hypertensive drugs in the 1960s and 1970s started this decline, and the creation of screening and treatment referral centers for high blood pressure may account for the marked decline in the late 1970s. The long-term follow-up on the Framingham Heart Study revealed that long-term stroke survivors, especially those with only one episode, have a good chance for full functional recovery.5 For people left with severe neurological and functional deficits, studies have demonstrated that rehabilitation is effective and that it can improve functional ability.6,7 It has been demonstrated that age is not a factor in determining the outcome of the rehabilitation process.8 Currently it is thought that clients should be given an opportunity to participate in the rehabilitation process, regardless of age, unless it is medically contraindicated. The prediction of ultimate functional outcome has been hampered by the inaccuracy of commonly used predictors (medical items, income level, intelligence, functional level). Computed tomography (CT), functional magnetic resonance imaging, and regional cerebral blood flow studies are used in diagnosis and increasingly as predictors of functional recovery after stroke. Positron emission tomography and single-photon emission CT are newer techniques that are used in research centers to define areas of dysfunctional but perhaps “salvageable” tissue.2,9 Atherosclerotic plaques and hypertension interact to produce cerebrovascular infarcts. These plaques form at branchings and curves of the arteries. Plaques usually form in front of the first major branching of the cerebral arteries. These lesions can be present for 30 years or more and may never become symptomatic. Intermittent blockage may proceed to permanent damage. The process by which a thrombus occludes an artery requires several hours and explains the division between stroke-in-evolution and completed stroke.10 The embolus that causes the stroke may come from the heart, from an internal carotid artery thrombosis, or from an atheromatous plaque of the carotid sinus. It is usually a sign of cardiac disease. The infarction may be of pale, hemorrhagic, or mixed type. The branches of the middle cerebral artery are infarcted most commonly as a result of its direct continuation from the internal carotid artery. Collateral blood supply is not established with embolic infarctions because of the speed of obstruction formation, so there is less survival of tissue distal to the area of embolic infarct than with thrombotic infarct.2 AV malformations are developmental abnormalities that result in a spaghetti-like mass of dilated AV fistulas varying in size from a few millimeters in diameter to huge masses located within the brain tissue. Some of these blood vessels have extremely thin, abnormally structured walls. Although the abnormality is present from birth, symptoms usually develop at ages 10 to 35 years. The hemorrhage of an AV malformation presents a pathological picture similar to that for the saccular aneurysm. The larger AV malformations frequently occur in the posterior half of the cerebral hemisphere.10 The focal neurological deficit resulting from a stroke, whether embolic, thrombotic, or hemorrhagic, is a reflection of the size and location of the lesion and the amount of collateral blood flow. Unilateral neurological deficits result from interruption of the carotid vascular system, and bilateral neurological deficits result from interruption of the vascular supply to the basilar system. Clinical syndromes resulting from occlusion or hemorrhage in the cerebral circulation vary from partial to complete. Signs of hemorrhage may be more variable as a result of the effect of extension to surrounding brain tissue and the possible rise in intracranial pressure. Table 23-1 summarizes the clinical symptoms and the anatomical structures involved according to specific arterial involvement. TABLE 23-1 Clinical Symptoms of Vascular Lesions Modified from Adams RD, Victor M: Principles of neurology, New York, 1981, McGraw-Hill. The frequencies of the three types of cerebrovascular disease—thrombosis, embolism, and hemorrhage—vary according to whether they were taken from a clinical study or from an autopsy study, but they rank in the order presented in this section. Ischemic strokes, thrombotic or embolic, account for 80% of strokes, and hemorrhagic strokes account for 20%.11 The clinical symptoms and laboratory findings for each type are condensed in Table 23-2. TABLE 23-2 Clinical Symptoms and Laboratory Findings for Neurovascular Disease—Ruptured Saccular Aneurysm CT, Computed tomography; TIA, transient ischemic attack. Modified from Adams RD, Victor M: Principles of neurology, New York, 1981, McGraw-Hill. Although infarcted tissue cannot at present be restored, medical management of the acute stroke from thrombosis or TIA is geared toward improving the cerebral circulation as quickly as possible to prevent ischemic tissue from becoming infarcted tissue. Cells that have 80% to 100% ischemia will die in a few minutes because they cannot produce energy, specifically adenosine triphosphate. This energy failure results in an activation of calcium, which causes a chain reaction resulting in cell death.1 Around this area of infarction is a transitional area where the blood flow is decreased 50% to 80%. Cells in the transitional area are not irreversibly damaged.12,13 One of the newer drugs available for immediate stroke treatment is tissue plasminogen activator (t-PA) (see Chapter 36). It is approved for use within 3 hours of symptom onset but is most effective if used within the first 90 to 180 minutes. Recent studies indicate that 42% of patients who have sustained a stroke wait 24 hours before getting care, with the average being 13 hours.13 The importance of community-wide programs to increase awareness of symptoms and effectiveness of emergency medical responses is immense for this drug’s usage. The American Heart Association and the National Stroke Association are creating community campaigns to increase awareness of the medical emergency nature of stroke symptoms. These campaigns encourage people to call 911 immediately when any of the following warning signs occur: Anticoagulant drugs are used to prevent TIAs and may stop a stroke-in-evolution. Before anticoagulant drugs are used, an accurate differential diagnosis is necessary because of the danger of excessive bleeding if hemorrhage is present. Heparin is often used in the early stage of the stroke, and warfarin (Coumadin) or dabigatran (Pradaxa) is commonly used in the months after the stroke. Cerebral edema, if present, is managed pharmacologically during the first few days. Antiplatelet drugs such as aspirin, dipyridamole (Persantine), and sulfinpyrazone (Anturane) are used to prevent clotting by decreasing platelet “stickiness.”10 Surgical treatment (thromboendarterectomy or grafting) is used when TIAs are the result of arterial plaques. Areas accessible to and suitable for surgery include the carotid sinus and the common carotid, innominate, and subclavian arteries. Although both surgery and anticoagulant therapy are used for TIAs, Adams and Victor10 extensively reviewed the wide divergence of opinions. For clients who have had a stroke yet recovered quickly and well, medical care focuses on prevention. Prevention usually includes maintaining blood pressure and blood flow, monitoring hypotensive agents (if given), and avoiding oversedation, especially for sleep, to prevent cerebral ischemia. Medical procedures for hypertensive hemorrhage parallel those for thrombosis and embolism. Surgical removal of the clot and lowering of the systemic blood pressure to decrease hemorrhage have generally not been helpful. Again, the preventive use of antihypertensive drugs in clients with essential hypertension is the soundest medical management available.10 Comatose clients are not good candidates for surgery. However, if the client survives the first few days and if the state of consciousness improves, surgical intervention, whether extracranial or intracranial, is the treatment of choice. Medical treatment consists of lowering arterial blood pressures. Bed rest for 4 to 6 weeks with all forms of exertion avoided is prescribed. Antiseizure medication may be used. Often a systemic antifibrinolysin is given to impede lysis of the clot at the site of rupture. Vasospasm, resulting in severe motor dysfunction, occurs with the use of drugs such as reserpine (Serpasil) and kanamycin (Kantrex) (see Chapter 36). Regardless of the cause of the stroke, comatose clients are managed by (1) treatment of shock; (2) maintenance of clear airway and oxygen flow; (3) measurement of arterial blood gases, blood analysis, CT, and spinal tap; (4) control of seizures; and (5) gastric tube feeding (if coma is prolonged). Hypertensive hemorrhage is one of the most common vascular causes of coma.14 Spasticity and its treatment constitute a major medical problem after stroke because clients complain about it, it may fluctuate, and it does not respond to one fixed treatment. The relationship between spasticity and movement after stroke is an area of continued interest for researchers. Recent studies have refuted the earlier belief that spasticity was inversely related to voluntary movement.15,16 Although therapists are more hesitant to treat spasticity now, physicians continue to treat it aggressively. Various pharmacological, surgical, and physical means are used to decrease spasticity. The pharmacological and surgical means are examined here, and therapy management is discussed later. Peripherally acting drugs are used to block a specific link in the gamma group. Procaine blocks selectively inhibit the small gamma motor fibers, resulting in a relaxation of intrafusal fibers. The effect of procaine blocks is transient. Intramuscular neurolysis with the injection of 5% to 7% phenol has been used to destroy the small intramuscular mixed nerve branches.17 Phenol blocks relieve hypertonicity and improve function, especially when followed by an intensive course of therapy.18 It can provide relief for 2 to 12 months, and the effects have been documented to last as long as 3 years.17,18 Disadvantages of phenol use include its toxicity to tissue and the complications of pain that occasionally result. Botulinum toxin type A (Botox) is also used to decrease the effects of hypertonicity on functional movement in hemiplegia.19–21 Local injection of the toxin into spastic muscles produces selective weakness by interfering with the uptake of acetylcholine by the motor end plate. The effect of the toxin is temporary, depends on the amount injected, and is associated with minimal side effects. Repeat injections are recommended no sooner than 12 to 14 weeks to avoid antibody formation to the toxin. Researchers report positive functional results when botulinum toxin A injections are followed by intensive muscle reeducation and appropriate splinting.22 Dantrolene sodium is used to interrupt the excitation-contraction mechanism of skeletal muscles. Trials have shown that it has reduced spasticity in 60% to 80% of clients while improving function in 40% of these clients. The side effects—drowsiness, weakness, and fatigue—can be decreased through titration of dosage. Serious side effects, including hepatotoxicity, precipitation of seizures, and lymphocytic lymphoma, have been reported when the drug has been used in high doses over a long time.17 Baclofen, in pill form, is used as a skeletal muscle relaxant to decrease spasticity. It can now be delivered intrathecally into the spinal cord with a pump that is surgically inserted into the body. It relieves spasticity with a small amount of medication (10 mg/20 mL, 10 mg/5 mL). Intrathecal baclofen has had dramatic results in cases of severe spasticity because it acts directly on the affected muscles instead of circulating in the blood. It is used for extremity spasticity that interferes with the ability to assume functional positions in patients with severe stroke, multiple sclerosis, head injury, and cerebral palsy.23 The highest risk for seizure after a stroke is immediately afterward; 57% of seizures occur in the first week and 88% occur within the first year.24 Seizures after thrombotic and embolic stroke are usually of early onset, whereas seizures after hemorrhagic stroke are of late onset. The management of seizures after stroke is usually with antiseizure medication. Commonly used drugs include phenytoin (Dilantin), carbamazepine (Tegretol), gabapentin (Neurontin), and divalproex (Depakote).25 Side effects that interfere with movement therapy include drowsiness, ataxia, distractibility, and poor memory. Fatigue is a major problem for the person with hemiplegia. This fatigability, which interferes with everyday life processes and active rehabilitation, is attributed to respiratory insufficiency resulting from paralysis of one side of the thorax. Haas and colleagues26 studied respiratory function in hemiplegia and found decreased lung volume and mechanical performance of the thorax to be significant factors, in addition to abnormal pulmonary diffusing capacity. Clients with hemiplegia consume 50% more oxygen while walking slowly (regardless of the presence or absence of orthotic devices) than that used by subjects without hemiplegia.26 The decreased respiratory output and the increased oxygen demand that result from atypical movement patterns are responsible for early fatigue in persons with hemiplegia. Treatment objectives and techniques must reflect the understanding of this respiratory problem. For clients who walk at velocities greater than 0.48 m/s, a gain in walking capacity is associated with an increased peak Vo2. Research exploring the role of exercise after stroke indicated that gains in respiratory fitness were associated with increased walking capacity. In clinical practice, therapists should remember to include standard respiratory measures and functions to evaluate the efficacy of treatment techniques.27
Movement dysfunction associated with hemiplegia
Overview
Epidemiology
Outcome
Pathoneurological and pathophysiological aspectsclassification
Thrombotic infarction.
Embolic infarction.
Hemorrhage.
Clinical findings

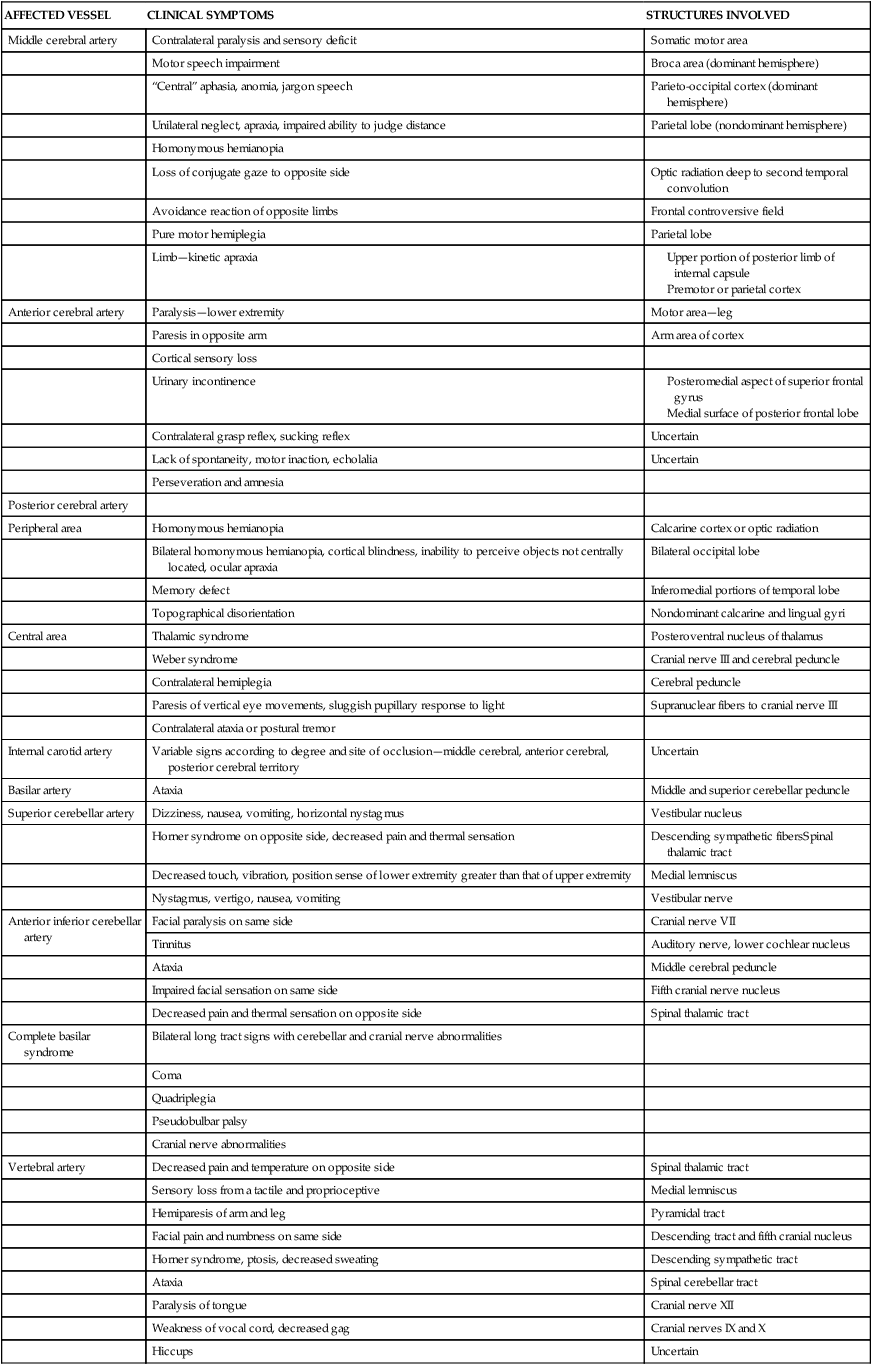
AFFECTED VESSEL
CLINICAL SYMPTOMS
STRUCTURES INVOLVED
Middle cerebral artery
Contralateral paralysis and sensory deficit
Somatic motor area
Motor speech impairment
Broca area (dominant hemisphere)
“Central” aphasia, anomia, jargon speech
Parieto-occipital cortex (dominant hemisphere)
Unilateral neglect, apraxia, impaired ability to judge distance
Parietal lobe (nondominant hemisphere)
Homonymous hemianopia
Loss of conjugate gaze to opposite side
Optic radiation deep to second temporal convolution
Avoidance reaction of opposite limbs
Frontal controversive field
Pure motor hemiplegia
Parietal lobe
Limb—kinetic apraxia
Anterior cerebral artery
Paralysis—lower extremity
Motor area—leg
Paresis in opposite arm
Arm area of cortex
Cortical sensory loss
Urinary incontinence
Contralateral grasp reflex, sucking reflex
Uncertain
Lack of spontaneity, motor inaction, echolalia
Uncertain
Perseveration and amnesia
Posterior cerebral artery
Peripheral area
Homonymous hemianopia
Calcarine cortex or optic radiation
Bilateral homonymous hemianopia, cortical blindness, inability to perceive objects not centrally located, ocular apraxia
Bilateral occipital lobe
Memory defect
Inferomedial portions of temporal lobe
Topographical disorientation
Nondominant calcarine and lingual gyri
Central area
Thalamic syndrome
Posteroventral nucleus of thalamus
Weber syndrome
Cranial nerve III and cerebral peduncle
Contralateral hemiplegia
Cerebral peduncle
Paresis of vertical eye movements, sluggish pupillary response to light
Supranuclear fibers to cranial nerve III
Contralateral ataxia or postural tremor
Internal carotid artery
Variable signs according to degree and site of occlusion—middle cerebral, anterior cerebral, posterior cerebral territory
Uncertain
Basilar artery
Ataxia
Middle and superior cerebellar peduncle
Superior cerebellar artery
Dizziness, nausea, vomiting, horizontal nystagmus
Vestibular nucleus
Horner syndrome on opposite side, decreased pain and thermal sensation
Descending sympathetic fibersSpinal thalamic tract
Decreased touch, vibration, position sense of lower extremity greater than that of upper extremity
Medial lemniscus
Nystagmus, vertigo, nausea, vomiting
Vestibular nerve
Anterior inferior cerebellar artery
Facial paralysis on same side
Cranial nerve VII
Tinnitus
Auditory nerve, lower cochlear nucleus
Ataxia
Middle cerebral peduncle
Impaired facial sensation on same side
Fifth cranial nerve nucleus
Decreased pain and thermal sensation on opposite side
Spinal thalamic tract
Complete basilar syndrome
Bilateral long tract signs with cerebellar and cranial nerve abnormalities
Coma
Quadriplegia
Pseudobulbar palsy
Cranial nerve abnormalities
Vertebral artery
Decreased pain and temperature on opposite side
Spinal thalamic tract
Sensory loss from a tactile and proprioceptive
Medial lemniscus
Hemiparesis of arm and leg
Pyramidal tract
Facial pain and numbness on same side
Descending tract and fifth cranial nucleus
Horner syndrome, ptosis, decreased sweating
Descending sympathetic tract
Ataxia
Spinal cerebellar tract
Paralysis of tongue
Cranial nerve XII
Weakness of vocal cord, decreased gag
Cranial nerves IX and X
Hiccups
Uncertain

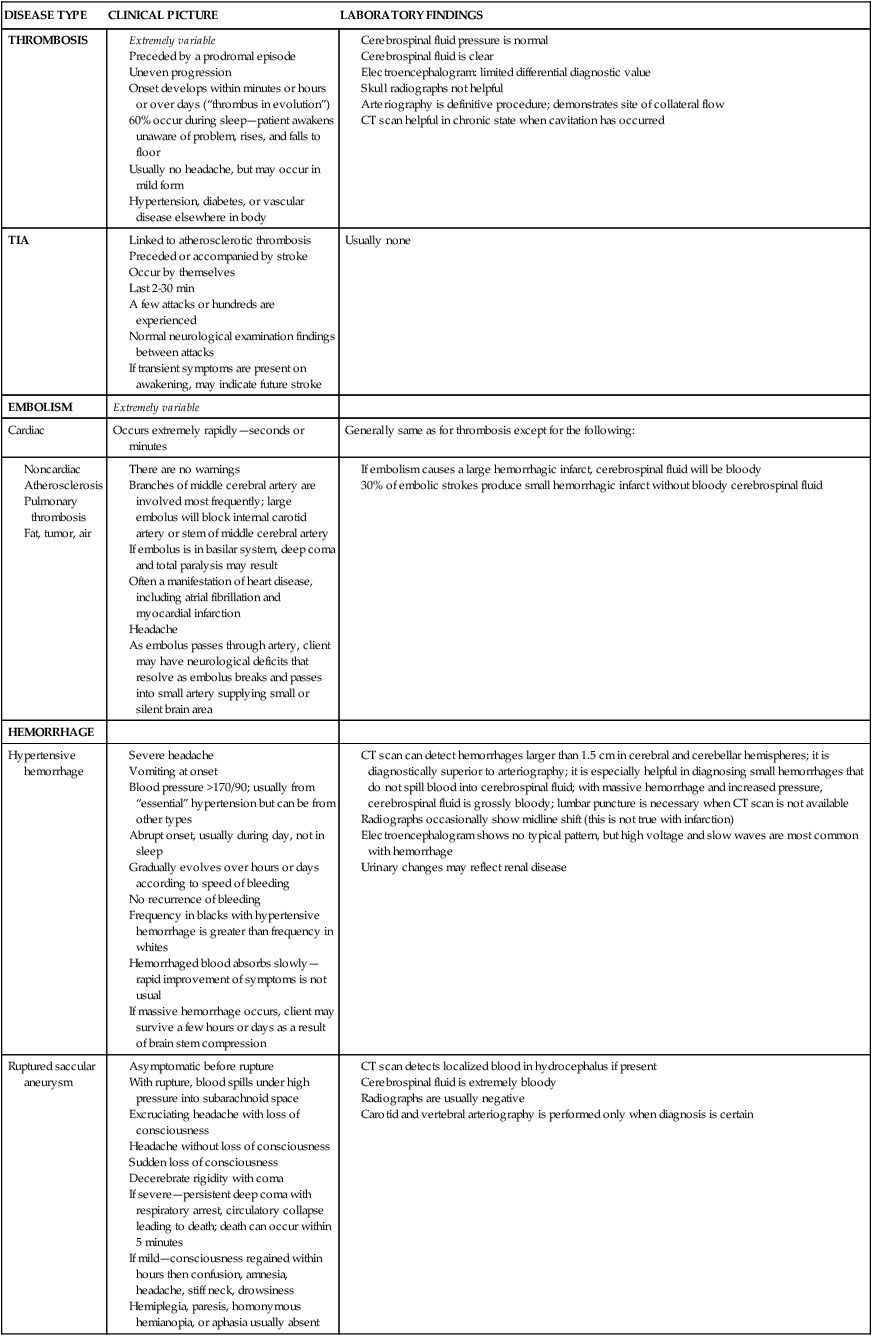
Medical management and pharmacological considerations
Acute medical care
Thrombosis and transient ischemic attacks.
 Sudden numbness or weakness of the face, arm, or leg, especially on one side of the body
Sudden numbness or weakness of the face, arm, or leg, especially on one side of the body
 Sudden confusion or trouble speaking or understanding
Sudden confusion or trouble speaking or understanding
 Sudden trouble walking, dizziness, loss of balance or coordination
Sudden trouble walking, dizziness, loss of balance or coordination
 Sudden severe headache with no known cause
Sudden severe headache with no known cause
Hypertensive hemorrhage.
Ruptured aneurysm.
Medical management of associated problems
Spasticity.
Seizures.
Respiratory involvement.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Movement dysfunction associated with hemiplegia
Only gold members can continue reading. Log In or Register to continue

