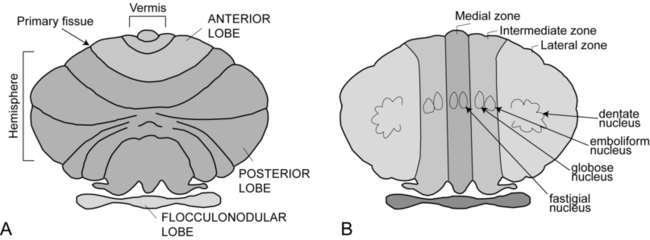
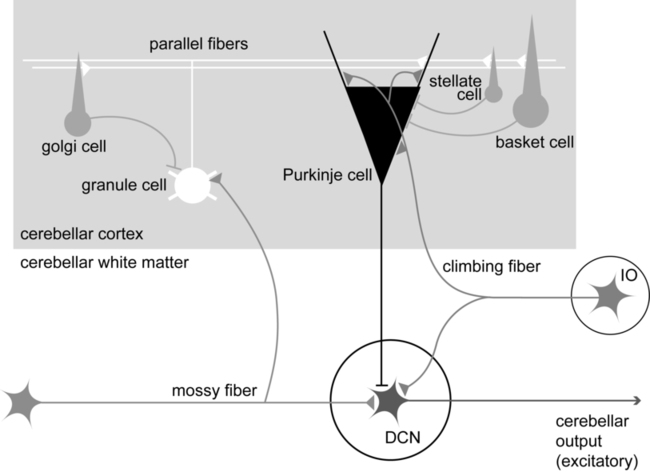
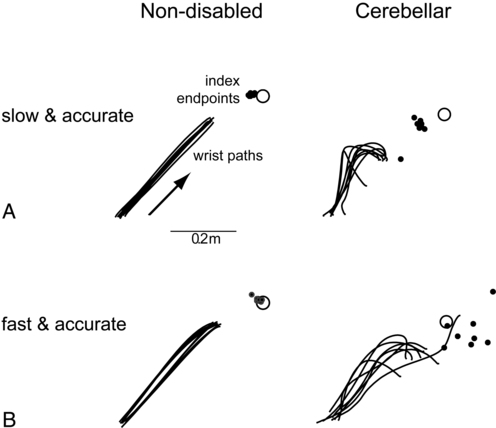
Cerebellar strokes are rarer than cerebral strokes, but not entirely uncommon. They account for less than 5% of all strokes.1 These strokes can involve any of the three arteries that supply the cerebellum: the superior cerebellar artery, anterior inferior cerebellar artery, and posterior inferior cerebellar artery. Depending on the territory supplied by the damaged vessel (Table 21-1), there are stereotyped patterns of cerebellar and extracerebellar motor dysfunction that result. However, there is certainly some variation in distribution from person to person. Stroke involving the superior cerebellar artery often leads to dysmetria of ipsilateral arm movements, unsteadiness in walking, dysarthric speech, and nystagmus.1 Stroke involving the anterior inferior cerebellar artery often causes both cerebellar and extracerebellar signs (owing to involvement of the pons) including dysmetria, vestibular signs, and facial sensory loss.1 Finally, stroke involving the posterior inferior cerebellar artery is usually, in the long run, the most benign, though initially it often manifests with vertigo, unsteadiness, walking ataxia, and nystagmus.1 The best predictor of recovery from cerebellar stroke is whether the deep cerebellar nuclei are involved: recovery is best when they are not damaged.2 TABLE 21-1 TERRITORIES OF THE CEREBELLAR ARTERIES Data from Amarenco P: The spectrum of cerebellar infarctions. Neurology 41:973–979, 1991; and Tatu L, Moulin T, Bogousslavsky J, Duvernoy H: Arterial territories of human brain: brainstem and cerebellum. Neurology 47:1125–1135, 1996. Tumors in the posterior fossa (i.e., in or near the cerebellum) do occur, though they are more common in children than adults. Depending on the type and location, tumors may be treatable with surgical resection, chemotherapy, radiation therapy, or some combination of these. Children with cerebellar tumors often have a good prognosis for recovery because many of the types of tumors most common in this population are benign and can be removed. Children also typically recover very well after cerebellar damage from tumor resection and show little signs of cerebellar ataxia. Tumors in adulthood often are caused by a more aggressive form of cancer and therefore may carry a poorer prognosis. Second to tumor type, damage of the deep cerebellar nuclei is an important factor that predicts recovery, even more so than age.2 Several neurodegenerative diseases can damage the cerebellum (Table 21-2). One of the more common types of degenerative diseases is a group of hereditary, autosomal dominant diseases referred to as the spinocerebellar ataxias (SCAs). Currently there are 30 known distinct SCAs, which are named by numbers (e.g., SCA1, SCA2). Depending on the genetic abnormality, they can cause either purely cerebellar damage or combined cerebellar and extracerebellar damage.3,4 Most of the SCAs have onset in midlife and are slowly progressive, which means that children of an affected parent will likely not know if they are affected until adulthood. There are genetic tests for a subset of these diseases. Because onset of symptoms is delayed and there are no effective pharmacological treatments, genetic counseling is a must before families decide whether or not to have children undergo genetic testing. A related set of diseases is the hereditary episodic ataxias,5 which are rare autosomal dominant diseases. As the name implies, clients with episodic ataxia will have periods of ataxia lasting minutes to hours, brought on by exercise, stress, or excitement. Some of the episodic ataxias respond well to medications.6 TABLE 21-2 SELECTED FORMS OF CEREBELLAR DAMAGE102 Data from Manto M, Marmolino D: Cerebellar ataxias. Curr Opin Neurol 22:419–429, 2009. Cerebellar damage can occur from other sources as well. In traumatic brain injury, damage of the cerebellum is almost always found in the presence of widespread brain damage and is seen as a predictor of poorer outcome.7 The cerebellum is also particularly sensitive to toxins, including certain heavy metals and alcohol. Chronic alcoholism causes cerebellar atrophy preferentially involving the anterior superior vermis.8,9 The inflammatory disorder multiple sclerosis also frequently produces lesions in the cerebellum. Finally, congenital brain abnormalities such as Chiari malformation damage the cerebellum by increased pressure and mechanical deformation. Recovery from cerebellar malformation is not understood; often these children have substantial damage to the brain stem or other neural structures, which may make therapy more challenging. A more comprehensive list of the variety of types of cerebellar damage is provided in Table 21-2. The cerebellum is part of the hindbrain and is positioned on the dorsal surface of the brain stem at approximately the level of the pons (Figure 21-1). It is connected to the brain stem by the superior, middle, and inferior cerebellar peduncles. The cerebellar peduncles contain all of the axons that transmit information to and from the cerebellum. The cerebellum can be anatomically divided into three lobes: the anterior, posterior, and flocculonodular lobes. The primary fissure divides the anterior and posterior lobes, and the posterolateral fissure divides the posterior and flocculonodular lobes (Figure 21-2). Looking at a sagittal slice through the cerebellum, distinct cellular regions can be visualized. The most superficial region is the cerebellar cortex, which, unlike the cerebral cortex, contains only three layers. The arrangement of cells within the cortex is strikingly uniform across all cerebellar lobes and plays a vital role in determining cerebellar function, which will be described later. Deep to the cerebellar cortex is the white matter layer, which contains the axons of Purkinje cells projecting out from the cerebellar cortex and the axons of mossy and climbing fibers entering the cortex from other brain and spinal regions (see Figure 21-1). The cerebellar nuclei are the output structures of the cerebellum, and they make up the deepest region. Groups of neuronal cell bodies receive information coming into the cerebellum from a variety of brain and spinal cord regions and also from the cerebellar cortex, via Purkinje cell axons. The deep nuclei are arranged in pairs, with one nucleus of each pair on each side of the cerebellum. Most medially are the fastigial nuclei, followed by the globose and emboliform nuclei and most laterally the broad dentate nuclei (see Figure 21-2). The medial and lateral vestibular nuclei also receive input directly from the cerebellar flocculonodular lobe and are therefore considered to play a role as an additional set of cerebellar output structures. Probably the most useful way of thinking about the anatomy of the cerebellum is to divide it into distinct functional longitudinal “zones.”10 Each cerebellar zone consists of a region of cerebellar cortex and its own pair of deep cerebellar nuclei. Each zone also has projections to and from distinct areas of the brain and spinal cord. Thus, despite the regular arrangement of cells over the entire cerebellum, each functional longitudinal zone is uniquely positioned to control certain types of movement but not others.10–12 See Table 21-3 for a summary. TABLE 21-3 FUNCTIONAL LONGITUDINAL CEREBELLAR ZONES The medial zone consists of the midline structure, the vermis, and the fastigial nuclei. This region of the cerebellum predominantly receives afferent information from the brain stem vestibular and reticular nuclei and the dorsal and ventral spinocerebellar pathways,13–18 which convey important information regarding the current sensorimotor state of the trunk and limbs.19–21 In turn, its outputs, through the fastigial nuclei, are largely to reticular and vestibular nuclei that will form part of the medial descending system (reticulospinal and vestibulospinal tracts), with some additional projections to the cerebral cortex via the thalamus.22–24 The medial cerebellar zone is involved in the control of posture and muscle tone, upright stance, locomotion, and in gaze and other eye movements. The intermediate zone is made up of the intermediate hemispheres and the globose and emboliform nuclei. This region also receives inputs from the dorsal and ventral spinocerebellar pathways and brain stem reticular nuclei, as well as some projections from the cerebral cortex that arrive via the cerebropontocerebellar pathway.11,13,14,25,26 Major projections from this cerebellar zone are to the cerebral cortex via the thalamus and to the red nucleus.23,24,27 The intermediate zone is considered to be important in controlling coordination of agonist-antagonist muscle pairs during a variety of activities including walking and voluntary limb movements. The medial and intermediate zones of the cerebellum are collectively referred to as the spinocerebellum, because these are the only cerebellar regions that receive afferents from the spinal cord. The largest region of the cerebellum is the lateral zone, which contains the two broad lateral hemispheres and their output structures, the dentate nuclei. Afferents to the lateral zone predominantly come from the cerebrum, from a wide variety of cortical areas including motor, premotor, and prefrontal cortices, parietal somatosensory and sensory association areas, and primary visual and auditory cortices.25,26 Outputs from the dentate travel mostly back to large areas of the cerebrum (through the thalamus), to many of the same areas from which afferents arrived in the cerebellum. Again, these include vast regions of sensorimotor cortices.27–33 Other efferent fibers project to the red nucleus in the brain stem. The lateral cerebellar zone plays a major role in control of complex, multijoint voluntary limb movements, particularly those involving visual guidance, and in the planning of complex movements and the assessment of movement errors. Because this region of the cerebellum interacts predominantly with the cerebrum, it is also commonly called the cerebrocerebellum. It is also sometimes referred to as the neocerebellum because it is considered to have arisen fairly recently in the phylogenetic tree, being much more expansive in primates than in lower animals.34 The flocculonodular lobe can be considered a fourth zone of the cerebellum. It receives afferent projections directly from the vestibular primary afferents (semicircular canals and otoliths) as well as from vestibular nuclei and visual brain regions.11,13,14,16,18 Outputs from the flocculonodular lobe project directly to the medial and lateral vestibular nuclei of the brain stem, without a synapse in a deep cerebellar nucleus.12,22,35 For this reason, these vestibular nuclei are sometimes considered an additional set of deep cerebellar nuclei. This cerebellar zone helps control eye movements and balance. The well known vestibuloocular reflex (VOR), which provides gaze stabilization during head turning or walking, relies on the cerebellum for proper functioning.36,37 Because of its critical ties to the vestibular system, the flocculonodular lobe is also known as the vestibulocerebellum (see Figure 21-2). Within a longitudinal zone, thousands of microzones may exist,11 each consisting of a highly organized group of connected cerebellar cortical neurons. A microcomplex is the name given to a neural circuit made up of a single microzone plus the other connected neurons with which it communicates directly. The following section provides a very brief overview of the circuits important for cerebellar function and reviews the flow of neuronal signals into and out of cerebellar microzones (Figure 21-3). Most afferent information enters the cerebellum through one of two pathways: the mossy fiber pathway or the climbing fiber pathway. Both have important actions on cerebellar Purkinje cells. The mossy fiber pathway affects “beams” or rows of Purkinje cells oriented along the cerebellar folia. Dense mossy fiber inputs arise from a wide variety of regions, including the cerebral cortex, several subcortical areas, the brain stem, and the spinal cord. Mossy fibers enter the cerebellar cortex and synapse onto granule cells, whose axons ascend and branch into parallel fibers. Each parallel fiber extends long distances longitudinally and synapses onto many Purkinje cells, all located along the same beam.38 Each parallel fiber has a relatively weak effect on single Purkinje cells, but the mass effect of many thousands of parallel fiber contacts with Purkinje cells drives the Purkinje cells to fire at high rates.39 In contrast, each climbing fiber arises exclusively from the inferior olive, located in the brain stem, and contacts only a few (approximately 1 to 10) Purkinje cells.12,40,41 Each Purkinje cell receives information from only one climbing fiber, yet the climbing fiber’s effect on the Purkinje cell is powerful, causing large complex spikes. The Purkinje cell provides the output for the cerebellar cortex; each Purkinje cell axon projects to one of the deep cerebellar nuclei. The mossy fiber and climbing fiber pathways affect Purkinje cells differently and are thought to transmit different types of information. Mossy fibers are active at very high rates (generating action potentials at approximately 100 Hz) and are highly modulated by various sensory stimuli and motor activity. They have been speculated to relay information related to the direction, velocity, duration, or magnitude of movements or sensory stimuli.42–45 Climbing fibers, however, are active at very low rates (approximately 1 to 4 Hz) and do not appear to be as strongly modulated by sensory stimuli or motor activity.40,46,47 There is still some disagreement regarding what sort of information is encoded in the climbing fiber signals, but the frequency of discharge appears to be too low to transmit information pertaining to specific parameters of sensory or motor events. The role of the climbing fiber is clearly important, however, because its firing produces large complex spikes in the Purkinje cells and can also powerfully affect subsequent Purkinje cell firing.41,48 One general theory states that a primary function of the cerebellum is in coordinating multiple limb segments to generate smooth and fluid multijoint movements.49–52 This “motor coordinator” theory has support from behavioral studies demonstrating that multijoint movements appear to be particularly impaired in clients with cerebellar lesions.50 Multijoint movements are inherently more complex than single joint movements because they require control of mechanical interaction torques; those occurring at one segment but caused by movement of other linked segments.53 This model suggests that the cerebellum predicts the mechanical interactions between segments based on a stored internal knowledge of limb dynamics, and helps generate the correct motor commands for appropriate multijoint movements. A second popular theory is the timer hypothesis. This idea proposes that the cerebellum is the main site for the temporal representation of movements.54,55 Supporters of this theory suggest that cerebellar output ultimately encodes the precise temporal sequence of muscle activation with such precision that a cerebellar lesion produces obvious deficits in the spatial domain (e.g., movement direction and magnitude) as well as the temporal domain.56 Other studies have shown that individuals with cerebellar damage also have impairments in perceiving time intervals, suggesting that this could be a more general cerebellar function.57,58 A third idea is that the cerebellum acts as an internal model to allow predictive control of movement. Sensory feedback is inadequate for movements that need to be both fast and accurate: it is too slow, and as a result, motor corrections would be issued too late. Instead, the brain generates motor commands based on an internal prediction of how the command would move the body. This “feed-forward” control requires stored knowledge of the body’s dynamics, the environment, and the object to be manipulated, and it is learned from previous exposure. The neural representation of this knowledge is referred to as an internal model,59–62 as it provides the ability to reproduce the effects of motor actions in the brain. The internal model theory for cerebellar function states that the cerebellum serves as the site of an internal model for movement. Accordingly, the incoordination of movement associated with cerebellar damage is a consequence of an inaccurate internal model, which disrupts nearly all aspects of feed-forward motor control.63 This idea is appealing, as it could help explain the wide variety of motor behaviors (e.g., reaching, standing balance, eye movements) and movement parameters (e.g., force, direction) that can be impaired after cerebellar damage. Likewise, human behavioral studies have recently pointed out that cerebellar damage is frequently associated with impaired feed-forward control but relatively intact feedback mechanisms.64,65 A related theory originates from the seminal works of Marr,66 Albus,67 and Ito,68 in which the cerebellum was theorized to be a sort of “learning machine.” This theory was based on careful examination of the anatomy and physiology within cerebellar microcircuits, and today it continues to provide the basis for many of the current theories of cerebellar function (i.e., those described earlier). Central to the idea of cerebellar involvement in learning was the discovery that Purkinje cell output can be radically altered by climbing fiber induction of long-term depression (LTD) of the parallel fiber–Purkinje cell synapse.48 Hence, climbing fiber inputs onto Purkinje cells can be viewed as providing a unique type of teaching or error signal to the cerebellum. More recently, LTD, long-term potentiation (LTP), and nonsynaptic plasticity have all been shown to exist at numerous sites within the cerebellum, both in the cortex and in the deep cerebellar nuclei.69–72 Thus there are multiple avenues for activity-dependent plasticity to occur within the cerebellum over relatively short time scales. It is presumed that the plastic changes in cerebellar output are responsible for changing motor behavior during the process of learning new skills. Ataxia is the primary sign of damage to the cerebellum or its input structures. Ataxia refers generally to uncoordinated or disordered movement, which, though most often associated with gait (gait ataxia), can also be used to describe uncoordinated arm or leg movements (limb ataxia). Ataxia is exacerbated by moving multiple joints together and by moving quickly. Because ataxia is a nonspecific term, it is important in both clinical and research settings to use more precise terminology to describe the specific aspects of motor performance that are impaired. Around the beginning of the twentieth century, Joseph Babinski and Gordon Holmes were two of the earliest investigators to describe many of these specific features we now discuss here.73–75 Dysmetria specifically refers to an impaired ability to properly scale movement distance. Movements are described as either hypermetric or hypometric, referring to overshooting or undershooting of targets, respectively. Many clients with cerebellar lesions will show both forms of dysmetria even during successive movements (Figure 21-4).49,50 Dysmetria can be seen in both proximal and distal joints and occurs during both single-joint and multijoint movements, though multijoint movements worsen dysmetria (Figure 21-5).49,76–78 Slow movements tend to produce hypometria, whereas fast movements almost always bring about hypermetria.49 For this reason, it has been speculated that hypometria represents more of a voluntary compensation for hypermetria than a primary impairment from cerebellar damage. Sometimes large end point errors can be reduced to some degree with visual feedback, but even the corrective movements themselves are still abnormal.79 One proposed mechanism for dysmetria is an impaired ability to predict and account for the dynamics of the limbs. In particular, clients with cerebellar lesions have been demonstrated to have a specific deficit in the ability to account for interaction torques,49,50 the rotational forces that act on a limb segment when another linked limb segment is in motion.68 When the cerebellum is intact, the central nervous system is able to predict the effects of interaction torques and appropriately counter or exploit them so as to produce a smooth, straight, and accurate reach in a feed-forward manner. When the cerebellum is damaged, an incorrect or absent accounting for interaction torques leads to an incorrect feed-forward motor plan and subsequently an uncoordinated, overly curved and hypermetric or hypometric reach that requires feedback corrections to reach the target location. Originally, Babinski80 coined the term asynergia to mean a deficit in the coordination of movements of one body region or in one limb segment with movements of another. Today, dyssynergia is used to describe impairment of multijoint movements, wherein movements of specific segments are not properly sequenced or of the proper range or direction, resulting in uncoordinated multijoint movement. As indicated earlier, it is nearly universally true that clients with significant cerebellar damage show greater impairments during multijoint movements than single-joint movements. However, it is not fully understood whether the reason for that is because the deficits of single-joint movements are compounded during a multijoint movement or because the cerebellum plays a special and unique role in multijoint control. Dyssynergia appears to be related to dysmetria and therefore is probably also related to a deficit in predicting limb dynamics.49 Dysdiadochokinesia specifically refers to a deficit in the coordination between agonist-antagonist muscle pairs elicited during voluntary rapid alternating movements.80 Such coordination is typically tested during performance of simple, fast alternating movements such as forearm supination-pronation or hand or foot tapping. Characteristic deficits are excessive slowness along with inconsistency in the rate and range of the alternating movements, which worsen as the movement continues.74 Dysdiadochokinesia appears to be caused by poor regulation of the timing of cessation of agonist muscle activity and the initiation of antagonist muscle activity,81,82 which could be related to a deficit in predicting limb dynamics. Indeed, rapid reversals in movement are dynamically difficult to control. Movement decomposition refers to the breaking down of a movement sequence or a multijoint movement into a series of separate movements, each simpler than the combined movement.75 An example of this is the well known finding that clients with cerebellar damage, when asked to reach to a target in front of and above the resting arm, will often flex the shoulder first and then, while holding the shoulder fixed, extend the elbow.49 This approach is generally slower and will produce a more curved trajectory of the finger to the target compared with nondisabled individuals, who would typically perform the shoulder flexion and elbow extension at the same time to produce a nearly straight-line finger trajectory. Most likely, decomposition reflects more of a compensatory strategy for dealing with impaired multijoint movements than it does a primary sign of cerebellar damage.49,83 Despite being a very common neurological sign, tremor is poorly defined and not well understood. There are several different forms of tremor, many with different causes, only some of which are related to cerebellar dysfunction, so it is important to distinguish among them. Tremor associated with damage to the cerebellum is typically called action tremor, reflecting the fact that it is absent at rest and elicited during muscle activation and distinguishing it from the resting tremor associated with Parkinson disease. Action tremor can be classified as postural or kinetic tremor.84 Postural tremor occurs in muscles maintaining a static position against gravity (e.g., holding arms out in front of the body or standing in place), whereas kinetic tremor occurs in muscles producing an active voluntary movement. Therefore the movement oscillations are most visible in the same plane as the voluntary movement. Kinetic tremor typically occurs at relatively low frequencies (approximately 2 to 5 Hz) and can be observed during simple nontarget-directed movements such as forearm pronation and supination or foot tapping, or during targeted movements such as pointing during the finger-to-nose test. Intention tremor is a specific form of kinetic tremor that occurs during the terminal portions of visually guided movements toward a target. It may actually represent the multiple corrective movements, driven by visual feedback, to reach the target. As such, intention tremor can be tested by repeating the test movement with eyes closed; if the tremor decreases substantially or disappears, it is an intention tremor.79 Classic cerebellar tremor is kinetic tremor with intention tremor at movement termination. In general, cerebellar tremor is thought to be caused by an insufficient ability to anticipate the effects of movement and excessive reliance on sensory feedback loops.85 Cerebellar tremor is highly influenced by sensory conditions and has a strong mechanical component; it is significantly reduced during isometric conditions or when vision is removed. It also can be decreased in some clients by adding an inertial load to the limb,86 though that strategy may also increase dysmetria.87 There may also be a significant central component to cerebellar tremor, possibly related to influences from the thalamus or the inferior olive.81,88 Hypotonia in clients with cerebellar damage was first described by Holmes.75 It appears to arise from decreased excitatory drive to vestibulospinal and reticulospinal pathways, two major output pathways from the cerebellar vermis and flocculonodular lobe. The hypotonia usually manifests as a decrease in the extensor tone necessary for holding the body upright against gravity. In cats, lesions of either the vestibular or fastigial nuclei cause this sort of postural hypotonia.51,89–91 More recent observations in humans indicate that hypotonia is typically most problematic in cases of severe cerebellar hypoplasias affecting the vermis, such as Joubert syndrome,92 or in adults during the acute stage of cerebellar injury only. In cases of adult-onset acute injury, hypotonia usually resolves naturally over time and clients recover normal passive muscle tone and normal reflexes quickly. Thus hypotonia typically presents minimal to no problems for physical function.81 Classically, cerebellar imbalance during stance was considered to be of a similar magnitude whether or not the eyes are open; that is, little improvement noted with visual feedback73,75 and a negative Romberg test result. However, more recently, investigators using posturography measures have been able to distinguish several different categories of cerebellar imbalance during quiet standing, some of which do show improvement with visual stabilization.93,94 For instance, clients with cerebellar damage relatively isolated to the anterior lobe typically show increased postural sway, which is of a high velocity and low amplitude and occurs mainly in the anterior-posterior dimension. These individuals also tend to have associated postural tremor and increased intersegmental movements of the head, trunk, and legs and tend to improve when allowed visual information. On the other hand, localized damage to the vestibulocerebellum more often leads to increased postural sway that consists of low-frequency and high-amplitude movements without a preferred direction and without increased intersegmental movements. These individuals typically show no improvement with visual information. Clients with damage limited to the lateral cerebellum tend to have only slight or even no postural instability at all.93–95 Human cerebellar damage is also associated with hypermetric postural responses to surface displacements or during step initiation; that is, dynamic instability.96,97 Specifically, clients tend to produce larger-than-normal surface-reactive torque responses and exaggerated and prolonged muscle activity, thereby overshooting the initial posture during the return phase of the recovery from a perturbation (Figure 21-6).
Movement dysfunction associated with cerebellar damage
Overview
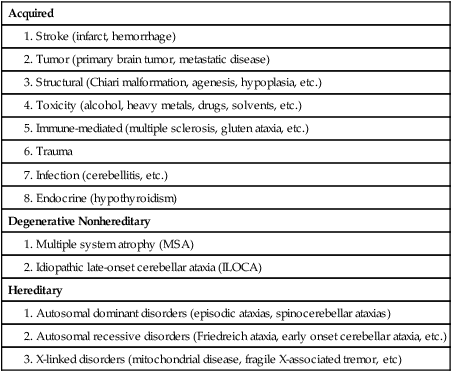
Types of cerebellar damage

ARTERY
CEREBELLAR TERRITORY SUPPLIED
SCA
Superior or upper half (approximately) of the dorsal and upper third (approximately) of the ventral surface of the cerebellum except for the extreme lateral wing of the hemisphere
Portions of the vermis and nodulus
Substantial upper portions of the intermediate and lateral hemispheres
Portions of the deep cerebellar nuclei
Superior cerebellar peduncle
AICA
Middle 10%-30% of the ventral cerebellum, sometimes wrapping laterally to encompass a small portion of the most lateral aspects of the dorsal cerebellum
Flocculus
Small portions of the lateral hemisphere
Middle and inferior cerebellar peduncles
PICA
Inferior or lower half (approximately) of the dorsal cerebellum and the inferior fourth to third of the ventral cerebellum
Portions of the nodulus, vermis, intermediate and lateral hemispheres
Portions of the deep cerebellar nuclei

Acquired
Degenerative Nonhereditary
Hereditary
Cerebellar anatomy and physiology
Anatomical divisions
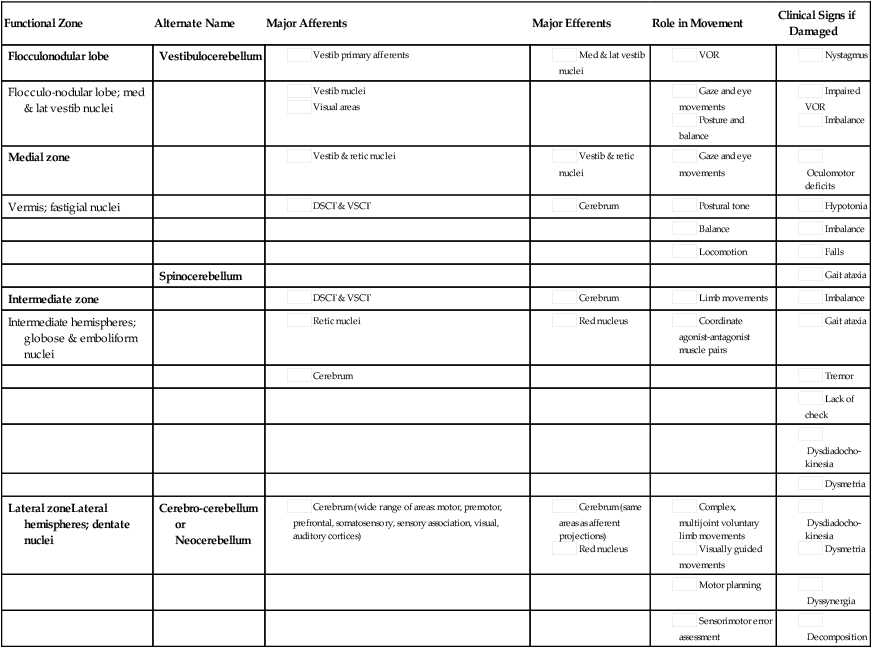
Functional divisions and their afferent and efferent projections

Functional Zone
Alternate Name
Major Afferents
Major Efferents
Role in Movement
Clinical Signs if Damaged
Flocculonodular lobe
Vestibulocerebellum
Flocculo-nodular lobe; med & lat vestib nuclei
Medial zone
Vermis; fastigial nuclei
Spinocerebellum
Intermediate zone
Intermediate hemispheres; globose & emboliform nuclei
Lateral zoneLateral hemispheres; dentate nuclei
Cerebro-cerebellum or Neocerebellum
Physiology of cerebellar neuronal circuits
Cerebellar function in adapting and controlling movement
Theories of cerebellar function
Clinical manifestations of cerebellar lesions
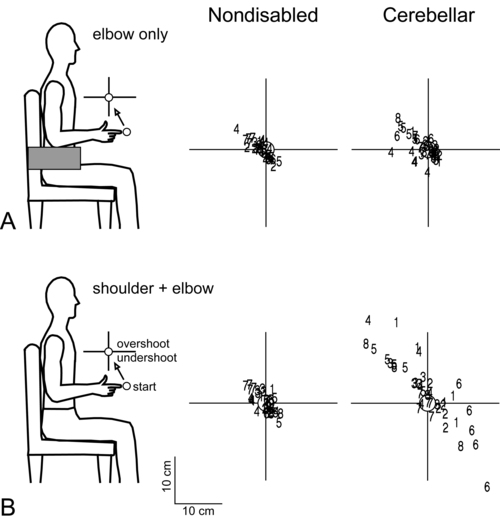
Dysmetria
Dyssynergia
Dysdiadochokinesia
Decomposition
Cerebellar tremor
Hypotonia
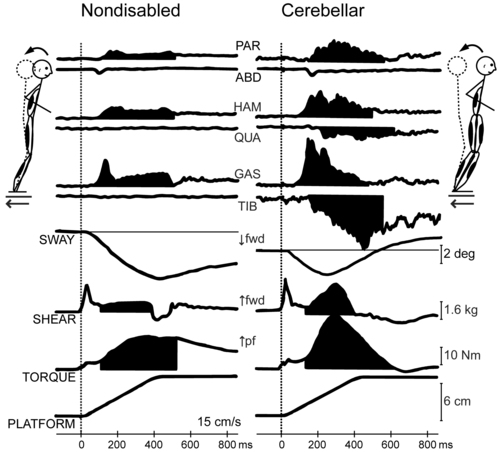
Imbalance
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Movement dysfunction associated with cerebellar damage
Only gold members can continue reading. Log In or Register to continue