Mosaicplasty/OATS
Emilio Lopez-Vidriero
Donald H. Johnson
INTRODUCTION
Osteochondral lesions have always been a challenge for orthopaedic surgeons. With the increasing use of arthroscopy, surgeons have been able to diagnose these lesions more often. However, the pathophysiology of osteochondral defects is unclear. Some defects are small and very painful, and others are not. These defects are probably due to the impaired ability of the joint surface to absorb energy and transmit load. Pain probably occurs because of the stimulation of nerve endings of the subchondral bone in these weight-loading surfaces.
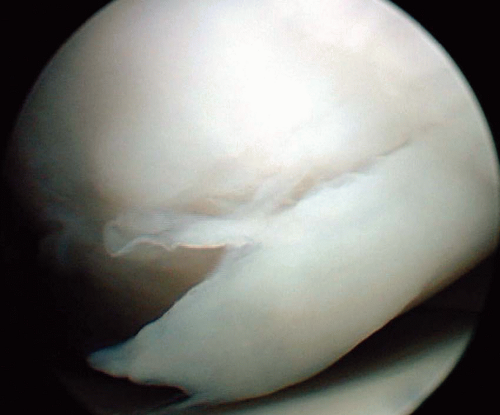
The rationale for treatment of this pathology is that chondral lesions can lead to further injury to the joint and ligaments, as the irregular surfaces alter biomechanics. Further, partial-thickness tears (chondral fractures) (Fig. 41.1) tend to not heal and full-thickness tears (osteochondral fractures) heal variably (Fig. 41.2A and B). Usually, fibrocartilage fills the healing surface and it has a predominance of type I collagen with inferior biomechanical properties. This is in contrast to normal hyaline cartilage formed by collagen type II.
Treatment possibilities range from neglect, débridement to leave a stable defect, microfractures, and autologous condrocyte implantation, to osteochondral transplantation with auto or allografts. As yet, there is no gold standard technique in the armamentarium of the arthroscopist to treat osteochondral defects.
The guiding principle of osteochondral transplantation is that the loss of articular cartilage is replaced with identical hyaline cartilage tissue with similar biomechanical characteristics.
INDICATIONS
The best candidate for osteochondral autologous transplantation (OATS) is the young (20-30 years), thin (<25 BMI) patient who suffers a symptomatic, traumatic, unipolar, small (<2 cm2), and type IV outerbridge chondral defect exposing subchondral bone (Fig. 41.2A and B), confirmed previously with an MRI.
However, most chondral defects are discovered during diagnostic or therapeutic arthroscopy for other purposes. The surgeon must consider this possibility in order to have the necessary tools ready. Chondral defects are more common in meniscectomized knees and ACL-deficient knees, both acute (33%) (1) and chronic (79%) (2). Other risk factors are PCL-deficient knees due to sheer force and malalignment of the lower extremity.
Originally, autologous osteochondral transplantation was limited to relatively small or medium-sized focal chondral and osteochondral defects of the weight-bearing surfaces of the femoral condyles and the patellofemoral joint. With time and experience, the indications have extended to other joints, including talar; tibial; humeral, both head and capitelar; and femoral head lesions.
The pathologies that are usually addressed with this technique are chondral defects, both traumatic (Fig. 41.2A) and degenerative (Fig. 41.2B); osteochondritis dissecans; and avascular necrosis. This is especially true if the knee is unstable.
The indications for osteochondral transplantation are limited mainly by donor-site availability and the biological healing response. Other technical circumstances also determine the indication for transplantation.
Practical considerations for successful transplantation suggest that the diameter of the defect should be between 1 and 4 cm2. Usually, both patellofemoral peripheries allow graft harvest for defects of 3 to 4 cm2 in size.
In certain cases where the deficiency of the loading site of the knee ranges from 6 to 9 cm2, it may be possible to use the posterior medial femoral condyle as the donor. This technique is called Mega-OATS (3) and it is an option worth considering until such time as total knee replacement is indicated in young adults in whom hyperflexion of the knee is not needed.
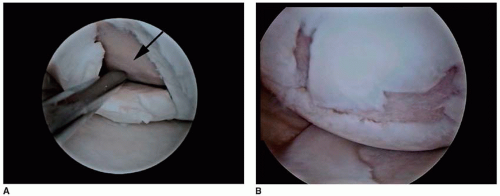 FIGURE 41.2 A: Traumatic chondral lesion. Note the full-thickness unstable flap with subchondral bone exposed. B: Degenerative complex chondral lesion with unstable flaps. |
However, the potential use of osteochondral allografts has expanded the indications, removing donor site availability as a problem. Allografts will be discussed later in this chapter.
The biological healing response is mainly limited by age. Most surgeons consider that 50 years of age is the recommended upper limit for the procedure, and this reflects clinical outcomes of single-block osteochondral transfer (4).
It should be noted that the indications for this treatment depend on other possible pathologies that the recipient joint could suffer. Thus, the treatment of instability, malalignment, and meniscal and ligament tears must be incorporated in the operative and postoperative rehabilitation algorithms.
CONTRAINDICATIONS
Formal contraindications for this type of treatment are as follows:
infections or tumor defects;
generalized acute and chronic arthritis, rheumatoid and/or degenerative in type;
delocalized uni/multicompartmental osteochondral lesions of the knee;
tricompartmental knee arthrosis;
chondrocalcinosis;
not simultaneously corrected or noncorrectable malalignment or ligamentous instabilities.
Relative contraindications are the following:
Age between 40 and 55
Mild osteoarthritic changes
Smokers
As stated before, patients over the age of 50 are usually not good candidates. Nor are those patients under the age of 50 with early unicompartment arthritis where the donor site cartilage is thin and the cartilage surrounding the defect is of poor quality. Smoking should be considered a possible contraindication due to its deleterious effects on the vascular tree, avoiding reparative tissue creation.
Technical difficulties make deep, large osteochondral defects unsuitable for osteochondral transplant, mainly because of the limited availability of autologous osteochondral grafts. Lesions deeper than 8 mm make it difficult to reconstruct the subchondral bone while achieving coverage and maintaining congruency of the transplanted cartilage surface. Also, it is difficult to reconstruct subchondral bone, restore the contour of the large defect area, and cover the entire defect area with hyaline articular cartilage.
TECHNIQUE
Cartilage transplantation can be performed either arthroscopically or open, including the miniopen technique. Some surgeons advise that those unfamiliar with the procedure should begin with the all open technique and after several cases, move to an arthroscopic procedure.
It is advisable to give intravenous antibiotics 30 minutes before the surgery. The type of anesthetic used, either general or regional, largely depends on patient preference. Both methods have been used with success. The application of a tourniquet is advised.
The patient’s position on the operating table should allow the knee to flex over 120 degrees for recipient side management. The contralateral extremity is usually placed in a stirrup or in lithotomy.
As with the diagnostic procedure, arthroscopy is begun through the anterolateral portal. In addition to other pathologies, the cartilage lesion is evaluated thoroughly. If the cartilage repair is to be performed, then portals are introduced more centrally than usual. If concomitant pathology is going to be addressed, usually the mosaicplasty procedure is performed last to avoid any possible malposition or movement of the plugs during the other procedures.
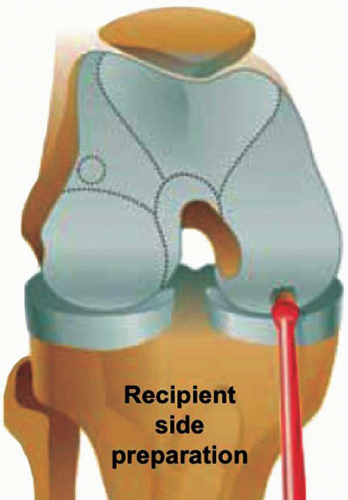
Defect Preparation
The edges of the lesion must be débrided until all unstable flaps are gone (Fig. 41.3A and B). The use of curettes is useful in obtaining clean edges, and the shaver is used to abrade the lesion to viable subchondral bone. The defect should be left surrounded by well-defined walls of stable normal hyaline cartilage to receive the plugs and stop progression of the lesion (Fig. 41.4).
The size of the lesion determines the number of plugs of different sizes that are needed to fill the gap. Generally, a gap filled to more than 70% is considered optimal, but rates can be increased up to 90% and 100% with variable-sized grafts (4,5).
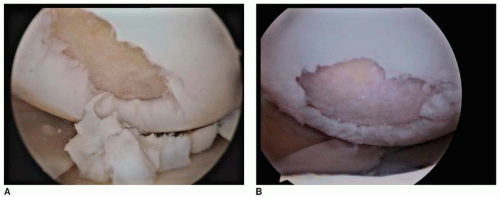 FIGURE 41.3 A: Débridement of the unstable flap and borders of traumatic tear seen in Figure 41.2A. B: Débridement of unstable flap of degenerative tear seen in Figure 41.2B. |
The size of the recipient side can be estimated by using a drill guide or sizers to measure the lesion on prepared subchondral bone. The various commercially available systems include different diameter tube harvesters, ranging from 2.7 to 14 mm, which are helpful in choosing the appropriate graft size. Subchondral bone can be marked with the harvesting chisels to give an approximate vision of the desired final result.
The aim is to achieve the best congruent filling and avoid gaps between the graft plugs that will be filled with fibrocartilage. There is a delicate balance to limiting the fill tissue between grafts, while optimally recreating a flush, congruent articular surface.
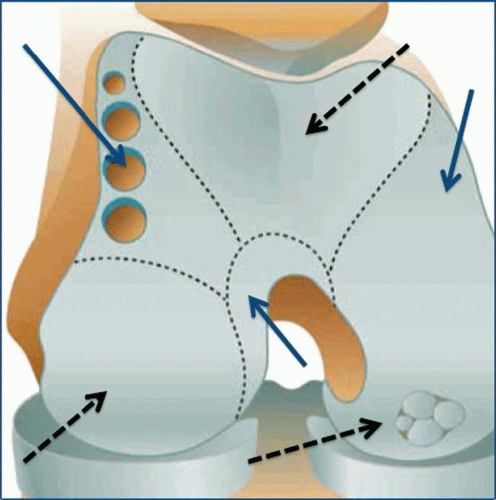
Donor Side Selection and Harvesting
There are several cartilage donor regions that may be chosen depending on their shape and congruency with the recipient side (Fig. 41.5).
Harvesting may be performed either arthroscopically or in an open fashion. Due to the importance of obtaining a perfectly perpendicular graft, the surgeon should decide whether to use one approach or the other. If arthroscopic harvesting is chosen, portal selection is crucial. The use of spinal needle before opening the portal is helpful in visualizing the possible orientation of the tube harvester that will be used next.
As long as care is taken not to damage healthy underlying articular cartilage or meniscus, portals may be made safely over much of the knee region.
A contralateral portal is advisable for viewing the portal site and ensuring a perpendicular alignment.
The knee may be extended to access more superior donor sites and then flexed progressively to reach the more inferior areas.
The sulcus terminalis of the femur is usually considered the inferior limit for graft harvest on both lateral and medial femoral condyles.
The originators of the technique suggested that the donor sites be at the periphery of the articular surface of the femur above the sulcus terminalis, including the lateral, medial, and superior edges (6). They studied ten different sites for harvesting and found that those exerting the least contact pressure were the superior lateral aspect of the lateral femoral condyle, followed by the superior medial aspect of the intercondylar notch.
All possible donor regions are important because each has a role in articular movement (7). Thus, harvesting is not free of consequences, but the least contact has been shown at the femoral condyles around the edge of the patella.
Because the patella is already displaced laterally with knee distension, making it easier to place the portal and do the harvest, some surgeons (4,5) prefer to use the medial border of the medial femoral condyle in the arthroscopic approach.
The shape of the donor site should also be taken into account depending on the recipient site. Normally, the periphery of the femur gives convex cores and the notch area produces grafts with concave articular surfaces.
Once the desired tubular graft harvester is chosen, it is introduced through the correct portal and placed perpendicularly over the selected donor cartilage (Fig. 41.6). If the superior condylar site is chosen for harvesting, the best way to control perpendicularity is to use the ipsilateral inferior portal for vision. When the harvester is in position, it is seated with the help of a mallet to a depth of 15 mm for chondral defects and 25 mm for osteochondral defects. This 10-mm difference provides additional cancellous bone in those defects that potentially need it. As the depth is important, visualization of the markings on the barrel must be clear to provide accurately sized grafts (Fig. 41.7). As a general rule, the graft should be two times longer than the diameter.
Once at the desired depth, the plug is released from subchondral bone. The different systems available have different release methods. The harvester may need to be toggled 5 degrees with no rotation (Smith and Nephew Mosaicplasty), or rotated 90 degrees clockwise and counterclockwise (Athrex OATS) (Fig. 41.7), or rotated 720 degrees (De Puy-Mitek COR), in order to fragment the cancellous bone at the base of the graft. The S&N system requires that the osteochondral graft be removed from the harvester. The graft is then pushed out from the osseous end to avoid cartilage injury and it is placed in a saline-filled basin. The Arthrex and De Puy-Mitek sets do not require free handling of the graft, as they remain in the harvester tube for transplantation.
Up to three grafts may be obtained from the standard portals. If more grafts are needed or if the surgeon prefers to go superiorly, superomedial or superolateral portals may be necessary. Additional grafts can be harvested by flexing or extending the knee.
Spacing the grafts’ harvesting to avoid confluence at depth (˜3 mm) will avoid any weakening of the condyle. The donor site holes will eventually fill with cancellous bone and fibrocartilage (2,4,8, 9, 10). Care should be taken when harvesting 6.5- and 8.5-mm grafts to avoid creating patellar tracking problems or weakening the condyle.
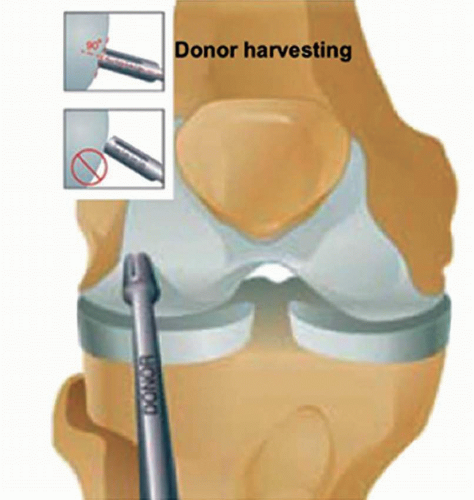 FIGURE 41.6 Donor site harvesting. Note that perpendicularity is critical for the success of the procedure. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree