Meniscus Repair: Inside-Out Technique
Charles R. Young
Richard D. Parker
Surgical treatment of knee meniscal pathology has experienced a dramatic shift in approach since its early recognition as a source of knee pain. Formerly thought of as vestigial structures, the menisci are now widely accepted to play a vital role in normal knee biomechanics, proprioception, and load transmission. As such, and with the maturation of arthroscopic techniques, the overall treatment scheme for meniscal disruption has shifted from that of complete excision to partial débridement and, whenever feasible, meniscal preservation. Techniques have evolved to allow reproducible healing of certain defects, which may ultimately avoid the premature development of degenerative knee disorders seen with meniscal volume loss and improve overall patient outcomes.
EPIDEMIOLOGY
Meniscal injury is one of the most common musculoskeletal injury patterns encountered in orthopaedic practice. Estimates of symptomatic incidence from 28 to 61 per 100,000 have been reported (4,9), making it more common than all other acute tendinous or ligamentous injuries. Under this umbrella of meniscal injury, two distinct patterns of injury can be seen that may have implications for optimal treatment and outcome.
One such pattern of injury includes a complex, degenerative pattern of meniscal disruption, with variable underlying articular cartilage injury. This includes horizontal cleavage and stellate tear patterns. These tears tend to occur more commonly in patients above age 30 to 35 and demonstrate increasing incidence with elevated body mass index and positive family history of knee osteoarthritis (14,34). Approach to these tears has largely evolved to include partial excision of the affected meniscus, with a relatively good prognosis for elimination of mechanical knee symptoms but a more guarded outlook for the maintenance of symptom-free knee function over the longer term due to progression of articular cartilage injury and degeneration.
An alternate pattern of injury includes acute traumatic tears, often involving the meniscal periphery. Many of these tears occur in younger and more athletic populations. Simple excision of the torn or displaceable meniscus would, in many cases, require the removal of a substantial percentage of meniscal volume. This holds demonstrated negative implications for the eventual development of degenerative joint disease (2,15,16,22,23,29,30,37,42,44). In select cases, meniscal preservation by way of repair can be attempted to meet the expectations of both returning patients to their normal activities and minimizing the impact of their injury on long-term knee health.
ANATOMY
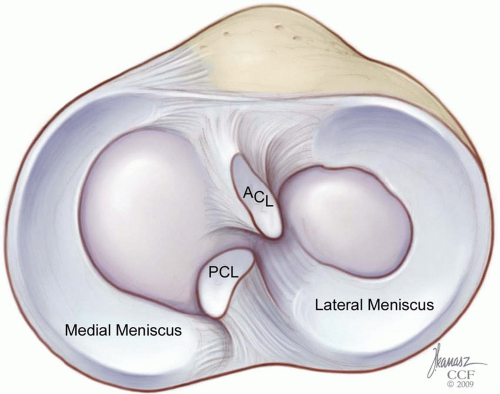
The menisci are wedge-shaped, semilunar structures that occupy the space between the distal femur and proximal tibia within the knee joint. They are thickest at their peripheral capsular attachments and taper centrally to provide a measure of congruity between the curved femoral condyles and relatively flattened tibial plateau (Fig. 22.1). This congruence is dynamic, with its peak in knee flexion, due to the flexibility and mobility of the menisci. The overall shape of the meniscus differs between the medial and lateral compartments. The medial meniscus has an inconsistent radius, being broader posteriorly, as well as an inconsistent radius of curvature as it extends a greater distance in the sagittal plane than in the coronal plane. This produces an overall “C”
shape, when viewed axially. The lateral meniscus has a more consistent radius throughout its curvature and its root attachments are situated nearer to each other in the sagittal plane. This produces a near complete “O” shape and, consequently, the lateral meniscus covers a greater percentage of the tibial articular surface within its compartment.
shape, when viewed axially. The lateral meniscus has a more consistent radius throughout its curvature and its root attachments are situated nearer to each other in the sagittal plane. This produces a near complete “O” shape and, consequently, the lateral meniscus covers a greater percentage of the tibial articular surface within its compartment.
The medial and lateral menisci differ in their attachment sites to the tibia, femur, peripheral capsule, and ligaments. As noted, the root attachments for the medial meniscus project both more anteriorly and posteriorly when viewed axially. The remainder of the periphery of the medial meniscus is solidly attached via its coronary ligament to the joint capsule. Additional contributing fibers from the deep portion of the medial collateral ligament anchor it medially and may provide an area of stress concentration, posterior to which many longitudinal tears may originate. By contrast, the lateral meniscus has no collateral ligament attachments and remains unattached to the peripheral capsule at its posterolateral corner, near the popliteal tendon hiatus.
Additional anatomic restraints exist in most individuals. A transverse ligament occurs in approximately two thirds of specimens, connecting the anterior horns of the menisci just anterior to the tibial insertion of the anterior cruciate ligament (ACL) (48,52). This is augmented in a minority of patients (˜15%) by anteromedial or anterolateral meniscofemoral ligaments, representing attachments from the anterior horns of the respective menisci to the opposing femoral intercondylar surface. The lateral meniscus is additionally restrained by two meniscofemoral ligaments. These attach the posterior horn of the lateral meniscus to the lateral aspect of the medial femoral condyle, by passing anterior (Humphry) and posterior (Wrisberg) to the posterior cruciate ligament (PCL). Variability in their presence has been reported in the literature; however, more recent reports demonstrate these as relatively constant structures (48,52). No such posterior meniscofemoral restraint occurs at the medial meniscus, which has been implicated in its higher injury rate. It remains unclear, however, whether these additional restraints play primary importance in meniscal stability or rather contribute to overall knee stability and proprioception.
At a microscopic and ultrastructural level, the menisci are composed of sparse fibrochondrocytes encased within a dense extracellular matrix of predominantly Type I collagen. This collagen is arranged into three distinct layers. The most superficial layer is composed of woven fibrils in a mesh-like pattern to provide smooth articulation with chondral surfaces and durability to surface disruption. Below this is a layer of random fibril orientation, which transitions to the deepest layer, composed of circumferential collagen fibrils cross-linked with radial tie fibers. The circumferential fibrils run longitudinally from root to root, generating hoop stresses as axial loads are applied through the knee joint. The cross-linking radial fibers serve as a scaffold for these longitudinal fibrils and help prevent propagation of longitudinal splits.
The remainder of the meniscal dry weight is composed of elastin and proteoglycan molecules. Glycoaminoglycoside side branches of these large proteoglycans account for approximately 1% to 2% of the meniscal mass (17). These molecules are strongly hydrophilic due to their net negative charge, which allows them to tightly bind water molecules. This assists the longitudinal collagen fibrils in resisting compressive forces applied across the meniscus
Aside from its structural organization, the menisci contain neurovascular elements unlike articular cartilage. Detailed studies of the perimeniscal capillary plexus have been undertaken, demonstrating penetration of these vessels into the peripheral meniscal bodies (3). These capillaries extend approximately 20% to 30% of the
radial width into the medial meniscus and approximately 10% to 25% into the lateral meniscus. They arise as distal arborizations from the respective geniculate vessel anastomoses. An additional vascularized synovial fringe extends 1 to 3 mm over the meniscal periphery but does not contribute to normal meniscal blood supply. The central majority of the meniscal bodies have no contributing vascular supply and derive nutrition via diffusion from the surrounding synovial fluid. This distribution has given rise to the red-white classification of meniscal zones. This variable blood supply has implications in the menisci’s ability to heal traumatic defects.
radial width into the medial meniscus and approximately 10% to 25% into the lateral meniscus. They arise as distal arborizations from the respective geniculate vessel anastomoses. An additional vascularized synovial fringe extends 1 to 3 mm over the meniscal periphery but does not contribute to normal meniscal blood supply. The central majority of the meniscal bodies have no contributing vascular supply and derive nutrition via diffusion from the surrounding synovial fluid. This distribution has given rise to the red-white classification of meniscal zones. This variable blood supply has implications in the menisci’s ability to heal traumatic defects.
Innervation of the menisci arises peripherally and at the root attachments. This includes proprioceptive fibers providing information on joint position and acceleration. Pain fibers also exist in the periphery, as probing or tension on the pericapsular meniscal tissue in awake patients causes a nociceptive response. The central portions of the meniscus lack this sensitivity.
MENISCAL BIOLOGY
Healing of meniscal tissue proceeds along the classic wound healing pathway of acute inflammation, development of granulation tissue, formation of a fibrovascular scar, and eventual maturation of fibrocartilage. This occurs more readily in a well-vascularized and mechanically stable setting. Experimentally induced tears within the vascular zone of the meniscus in a dog model demonstrate expected perimeniscal vascular invasion and mesenchymal cell proliferation. These defects showed mature healing by 10 weeks following injury (24).
Rather than being dormant spectators, meniscal fibrochondrocytes seem to be active in the healing process. When appropriately stimulated by growth factors, as may occur following a tear, they have the capacity to proliferate and synthesize extracellular matrix (24,31,49). In addition, superficial zone cells have the capacity to express α-smooth muscle actin, causing wound contraction. These properties seem to be independent of blood supply and indicate an intrinsic healing potential of the meniscus, if appropriately stabilized.
BIOMECHANICS
The menisci function in knee joint congruity and load transmission. Utilizing hoop stresses created by its longitudinal fibril orientation, the medial meniscus transmits approximately 50% of the overall medial compartment load. The lateral meniscus transmits up to 70% due to the greater percentage of coverage it provides to the lateral tibial plateau (47). These loads increase with knee flexion, from approximately 50% at full extension to nearly 90% at 90-degree flexion. Increasing knee flexion also shifts the distribution of force more posteriorly, increasing stress on the posterior horns leading to mechanical susceptibility (26).
Changes in load transmission are well documented in settings of partial or complete meniscectomy. Contact area on the femoral condyle decreases 50% to 70% following partial meniscectomy (25). Conversely, contact stresses can more than double. These alterations in joint contact pressures following complete or partial meniscal excision are thought to be responsible for changes seen to the articular cartilage over the long-term.
The medial meniscus has an additional role of providing secondary restraint to anterior tibial translation. This is most relevant in ACL deficiency. In this instability state, the posterior horn of the medial meniscus acts as a wedge to prevent anterior tibial translation, leaving it susceptible to injury. Forces recorded across the meniscus increase to 126% of normal at 30-degree knee flexion and 115% of normal at 90-degree knee flexion (32). The lateral meniscus does not contribute significantly to anterior knee stability.
SUITABILITY FOR REPAIR
Not all meniscal tears should be considered for repair. Tear location, type, size, tissue quality, patient age, compliance, and other factors are all important considerations when deciding upon treatment options. The method of treatment must also be selected based on surgeon technical preference and injury characteristics.
Tear location is of primary importance in assessing the healing potential of a meniscal defect. The red-red zone, within 3 mm of the meniscal periphery, is the optimal location for repair due to its well-developed blood supply. The red-white zone, within 3 to 5 mm, has some limited vascularity and an intermediate ability to heal. The white-white zone represents the central portion of the meniscus, >5 mm from the periphery. Tears in this area have no blood supply and have a significantly lower chance of healing (10,28). A watershed area also exists near the popliteal hiatus in the posterior lateral meniscus, as it is devoid of capsular attachment in this region (3). Certain patient characteristics, however, such as young age or high-level athletes, may prompt an attempted repair in a less desirable zone for healing despite the reduced chance of success when the alternative would be substantial meniscal resection.
Circumferential location of the tear may be important in the type of repair chosen. The majority of tears that are suitable for repair are found in the posterior and middle bodies and are excellent candidates for an inside-out technique. Anterior horn tears are often addressed more effectively with an outside-in approach. Those that involve the posterior one third and meniscal root may require all-inside or transosseous repair techniques to avoid injury to posterior neurovascular structures.
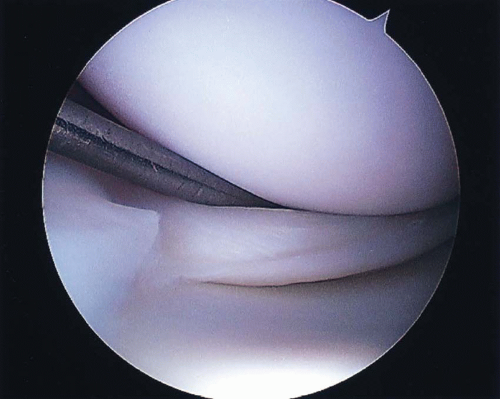 FIGURE 22.2 Unstable longitudinal medial meniscus tear originating near the attachment of the medial collateral ligament. |
Tear type is also an important factor. Longitudinal tears (Fig. 22.2), which extend vertically through the meniscus from its femoral to tibial surface, are the most amenable to repair because the tear margins can reliably be brought into close apposition and maintained with transcapsular sutures (12). Radial, parrot-beak, flap and horizontal cleavage tears are not able to be stabilized in the same manner, due to the disruption of circumferential collagen fibrils. Despite some success in repair of radial tear variants, these tear types should be considered for partial excision. Stable (<3 mm central displacement) or incomplete tears (involving only one surface of the meniscus) generally do not require repair. This is especially true for tears <5 mm in length or stable tears identified at the time of ACL reconstruction (50,51).
Tissue quality should be normal at the tear margins. Significant mucoid degeneration or chondrocalcinosis may indicate an unfavorable environment for healing and may be better served by partial excision. Patients older than 30 to 35 years of age may also have decreased expectations for successful healing, especially in the setting of significant articular cartilage disease. No definite recommendations exist for meniscal repair in the setting of diabetes, vascular insufficiency, inflammatory arthropathies, or connective tissue disorders. These are probably best approached on an individual basis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree