6
Microfracture of Articular Cartilage
Full-thickness defects of articular cartilage in the knee have a poor capacity for repair due to the lack of blood supply in hyaline cartilage. These lesions rarely heal spontaneously regardless of their etiology.1–6 The natural history of articular cartilage defects is the progression to osteoarthritis. If unchecked, these lesions may go on to develop profound arthritis that will ultimately require a total joint arthroplasty for pain relief.1 A variety of surgical techniques have been developed for the treatment of these lesions. Interventions include simple lavage and debridement, abrasion, drilling, osteochondral autografts, allografts, and autogenous cell transplantation.1, 2, 4, 7, 8 Dr. Richard Steadman originally developed the technique of microfracture about 20 years ago. The procedure attempts to enhance chondral resurfacing by stimulating the underlying marrow and providing an enriched environment for tissue regeneration through the use of the body’s natural vascular response to injury.4–7, 9 The microfracture technique allows blood to fill within the full-thickness chondral defects and quickly organize into a fibrous clot. Marrow elements, including mesenchymal stem cells, growth factors, fibrin, and platelets, become trapped in the defect. These cells undergo metaplasia to produce a reparative granulation tissue within the defect.3, 6, 10 Gradual fibrosis of the reparative tissue occurs over the ensuing days after surgery, and the fibrous tissue undergoes a progressive hyalinization and chondrification to ultimately produce a fibrocartilaginous mass that “heals” the defect. Steadman et al5, 6, 9 have performed this procedure on over 1800 patients and have reported predictably good results with slow improvement in patient function over a period of 2 years.
Surgical Indications and Other Options
Microfracture is indicated for both traumatic and degenerative lesions of the knee that have progressed to full-thickness chondral defects. In general, the technique is best indicated for full-thickness articular cartilage defects located in either a weight-bearing area between the femur and the tibia or in an area of contact between the patella and trochlear groove.4–7 Microfracture may also be used to treat unstable cartilage lesions that overlie the subchondral bone. The technique may be used for unipolar lesions that are present on only one side of the joint, as well as “kissing” (bipolar) lesions, which are located on both sides of the contacting joint surfaces. Although smaller (400 mm2), acute (12 weeks from injury) femoral and trochlear lesions have the most predictable results, there are no absolute contraindications to the technique based on the size or location of the lesion.3
General considerations including patient age, activity level, and acceptable biomechanical alignment of the knee are integral to appropriate patient selection. Patients being considered for the procedure should be relatively young (without significant global articular cartilage wear) and have a reasonable level of activity (able to tolerate a strict and rigorous rehabilitation protocol).5 Acceptable biomechanical alignment of the knee is essential for the success of the procedure, and preoperative weight-bearing alignment radiographs are a crucial part of preoperative planning.5 Correction of malalignment or patellar tracking abnormalities should be performed in conjunction with the microfracture procedure.
Relative contraindications to microfracture include chondral defects greater than 5 to 10 mm deep, and the presence of a malaligned limb with greater than 5 degrees of excess varus or valgus compared with normal. Other relative contraindications include any immune-mediated disease, disease-induced arthritis, and cartilage disease.3, 5 Other options to the microfracture technique depend on the size, location, and nature of the cartilage defect. In general, other available options include simple lavage and debridement, abrasion, drilling, osteochondral auto-grafts transplantation, autogenous cell transplantation, osteochondral allograft transplantation, correctional osteotomies, unicompartmental knee replacements, and total joint arthroplasty.1, 2, 4, 7, 8
Surgical Techniques
An initial thorough diagnostic arthroscopy of the knee is performed utilizing a standard 10-point examination. The surgeon should pay particular attention to the posterior aspects of the medial and lateral femoral condyle as well as the undersurface of the patella and trochlear groove. Any other necessary intraarticular procedures are typically performed prior to doing the microfracture. All articular surfaces are then carefully probed to assess the quality of the cartilage. Full-thickness fissures and flaps are assessed for degree of detachment, and all loose or marginally attached cartilage is debrided using an arthroscopic shaver (Fig. 6-1). The debridement should be taken back to a stable perpendicular edge so that a rim of healthy viable cartilage surrounds the defect. This stabilized defect provides a pool to hold the marrow clot as it is transformed into the fibrous “superclot.”5, 6
After the lesion is debrided, a curette is used to remove the calcified cartilage layer that remains as a cap to the base of the full-thickness defect. If a shaver is used during this portion of the procedure, bone removal needs to be carefully controlled to avoid violation of the subchondral bone. Basic science research has confirmed the importance of removing this calcified cartilage layer.4, 7 The percentage of the defect that is filled is enhanced with removal of the calcified layer, presumably due to providing a better surface for the “superclot” to adhere to. In addition, removal of the calcified zone may improve the nutrition of the repair tissue by expediting the diffusion of nutrients from the subchondral circulation.
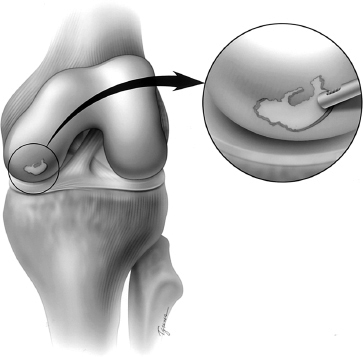
Figure 6-1 A full-thickness chondral defect on the medial femoral condyle. The defect is first debrided to remove all unstable cartilage flaps back to a perpendicular stable rim.
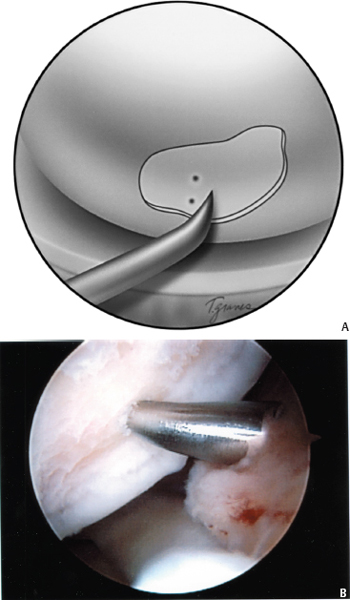
Figure 6-2 (A, B) After the calcified cartilage layer is removed, the prepared lesion is microfractured with an awl.
Once the defect has been prepared, a surgical awl (Linvatec, Largo, FL) is used to make multiple small holes (“microfractures”) in the exposed bone of the chondral defect (Fig. 6-2). The holes should be placed as close as possible, taking care not to connect adjacent microfractures. The holes will generally be spaced about 3 to 4 mm apart when this principle is adhered to (Fig. 6-3). The appropriate depth of the microfractures is confirmed with visualization of fat droplets from the marrow cavity. The microfracture method is preferred because it creates essentially no thermal necrosis of the bone compared with hand-driven or motorized drills. In addition, it provides controlled depth of penetration and allows access to difficult areas of the articular surface. The outer margin of the lesion is generally microfractured first. Care is taken to ensure that the awl penetrates the most peripheral aspects of the lesion so as to aid the healing of the repair tissue to the surrounding healthy, stable cartilage rim. Once the area has been microfractured, the prepared surface is roughened to promote adherence of the ensuing blood clot that contains the undifferentiated mesenchymal cells from the subchondral bone. The arthroscopic pump is turned off so that the flow of marrow fat and blood from the microfracture holes can be observed filling the defect under direct visualization.
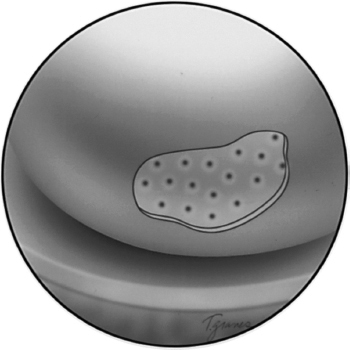
Figure 6-3 At the completion of the microfracture procedure, a series of microfracture holes should be spaced about 3 to 4 mm apart. Care needs to be taken not to penetrate the sidewall of an adjacent hole.
Tips and Tricks
Several surgical principles should be adhered to for optimal postsurgical results from the microfracture procedure. First, it is imperative for the surgeon to recognize and correct for any mechanical malalignment across the knee joint. Excess varus with an associated chondral defect on the medial femoral condyle or excess valgus with a concomitant lateral femoral condyle defect will place increased contact pressures across the microfracture repair site. Similarly, lateral patellofemoral lesions in the setting of patellar maltracking will have an increased likelihood of failure. By unloading the affected areas through high tibial osteotomy or patellar realignment at the time of the picking procedure, the lesion will be protected from these loads. Second, it is important to thoroughly debride the defect back to a stable rim. If flaps of nonviable cartilage remain overlying the microfracture site, the most peripheral aspects of the lesion will not be accessible. These flaps have the propensity to propagate, resulting in extension of the lesion and failure of the microfracture.
Finally, elimination of the calcified cartilage zone is extremely important to the success of the procedure. The calcified zone functions as an efficient barrier to cellular invasion. In the adult, little if any nutrient is able to diffuse across the tidemark due to heavy deposition of apatites in the calcified zone. Removal of this zone enhances both adherence of the marrow clotting elements and maturation into the fibrocartilage repair tissue. The general key to the procedure is to potentiate the transformation of the marrow clot, consisting of mesenchymal pluripotential stem cells, into a stable tissue within the lesion.5, 6, 9
Perhaps equally important as the surgical technique is the postoperative management. The goal of an appropriate rehabilitation program is to protect the repair site and promote the ideal physical environment in which the newly recruited mesenchymal stem cells can differentiate into the appropriate fibrocartilage cell line.5 Unlike the typical rehabilitation following debridement and drilling procedures, patients are kept at protected weight bearing for 6 to 8 weeks. Lesions on the weight-bearing surfaces of the femoral condyles should be limited to crutch-assisted touchdown weight-bearing ambulation for 6 to 8 weeks, depending on the size of the lesion.5 Patellar and trochlear groove lesions may be weight bearing as tolerated in a hinged brace with a 30-degree flexion stop. This prevents excessive pressure in the patellofemoral joint, because the patella does not engage the trochlear groove until after 30 degrees of flexion.3
Use of the continuous passive motion (CPM) machines should commence immediately in the recovery room.4, 5, 7 Rodrigo et al8 have documented the benefits of postoperative CPM after microfracture, and patients should be sent home with a CPM machine that they should use for 8 weeks. Patients come out of their brace when they are not weight bearing and go into a CPM machine from 10 to 90 degrees for at least 8 hours per day (generally at night). If they are unable to use a CPM machine, they are instructed to cycle their knee over the edge of a table 1500 times per day.
Active range-of-motion (ROM) exercises may begin gradually following the 6- to 8-week period of protected weight bearing with eventual progression to full weight bearing. No cutting, twisting, or jumping sports are allowed until at least 4 months postoperatively.3
Pitfalls and How to Avoid Them
Strict attention to surgical recommendations outlined above will eliminate most potential pitfalls. Several points warrant further discussion. First, removal of the calcified cartilage layer should generally not be performed with a shaver. Shavers are more difficult to control and may result in violation of the subchondral bone. If the subchondral bone is removed, the normal contour of the joint surface shape is lost, which compromises the knee joint congruity and the ultimate success of the procedure.3, 5 Second, the microfracture holes should be made perpendicular to the surface of the joint. Various angles and sizes of awls are available, and should be taken advantage of during the procedure. If the awl is not placed perpendicular to the surface of the bone, there is increased risk of penetration of the sidewall of a neighboring microfracture hole. Collapse of adjacent microfracture holes into one another may result in loss of structural integrity of the bony surface, and again, subsequent loss of knee joint congruity.
Some patients present with mild transient postoperative pain, most frequently after microfracture of the patellofemoral joint.5 These patients may complain of grating or gritty sensations with knee motion, secondary to small changes in the articular surface. Other patients may complain of more dramatic catching or locking as the apex of the patella rides over the steep perpendicular rim of the debrided lesion. Typically these symptoms resolve over a period of days to weeks, but may require an additional period of limited weight bearing and activity in a select few patients.11
Results (unpublished data) were reviewed for the Hospital for Special Surgery for isolated chondral defects of the medial femoral condyle. Nineteen patients were studied at a mean follow-up of 3 years. The mean size of the chondral defect was 3.2 cm2. Subjectively, 74% had minimal or no pain, and 63% rated their overall condition as good or excellent using the modified Cincinnati questionnaire. Objectively, one patient had swelling, and all patients had either mild or no crepitus on examination. Follow-up magnetic resonance imaging (MRI) was performed on all patients employing a special cartilage sequence to determine the intensity and morphology of the reparative fibrocartilage, bone edema, bony overgrowth, interface with the adjoining cartilage, percent fill of the defect, and appearance of the adjacent and opposing surfaces. Despite the good subjective results achieved, only 42% of patients had 67 to 100% fill of the defect on MRI, 21% had 31 to 66% fill, and the remaining 37% only had 0 to 30% fill. There was no correlation between the size of the defect and the percent fill. Four patients had a smooth transition at the fibrocartilage/articular cartilage interface, whereas the rest had a fissure.
Steadman and colleagues reviewed the results of the microfracture technique in over 100 patients at an average follow-up of 6 years. These results are reviewed by Gill.3 In this series, microfracture resulted in statistically significant improvement (p .05) in pain, swelling, and all functional parameters studied. The ability to walk 2 miles, descend stairs, perform activities of daily living, and do strenuous work and strenuous sports all demonstrated significant improvement. Of note, improvement in symptoms of pain and swelling continued up to 2 years postoperatively, and maximum functional improvement was not achieved until 2 to 3 years postoperatively.
Conclusion
The microfracture technique is a cost-effective and technically feasible procedure available to all surgeons who perform arthroscopy of the knee. Recent outcome studies have demonstrated its effectiveness in providing symptomatic and functional relief.3, 5–7 It is a reasonable first approach to the treatment of chondral defects, as it does not eliminate the potential to perform additional procedures such as a osteochondral autograft transplantation or autologous chondrocyte transplant should the microfracture fail.
The key to the success of the microfracture technique is dependent on the establishment of an optimal environment for the differentiation of pluripotential mesenchymal stem cells. This environment includes a source of marrow cells, provision of a matrix, removal of stress concentration, an intact subchondral plate, and some mechanical stimulation. Several factors affect the quality of the cartilaginous repair tissue in a full-thickness chondral defect treated by microfracture. First, the lesion must be debrided back to a stable rim. Second, the calcified cartilage layer must be removed without violating the underlying subchondral bone. Third, the subchondral bone must be penetrated by the awl with a 3 to 4 mm spacing of the holes to allow the superclot to fill the defect and adhere to the base of the defect. Fourth, a strict postoperative rehabilitation regimen must be adhered to, including immediate CPM and protected weight bearing. Finally, abnormal mechanical axes should be corrected at the time of the microfracture procedure. If these basic principles are followed, there is good potential for the formation of high-quality fibrocartilaginous tissue (Fig. 6-4) and ultimately, significant improvement in patients’ subjective and functional complaints.
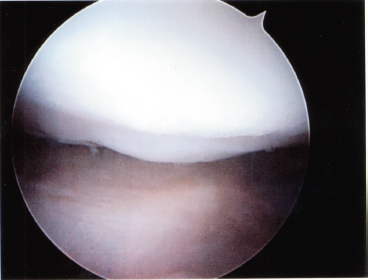
Figure 6-4 Second-look arthroscopy 5 months status post-microfracture surgery on a full-thickness chondral defect. Evaluation of the surgical site demonstrated high-quality fibrocartilaginous tissue overlying the microfractured defect.
References
1 Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–895
2 Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther 1998;28:203–215
3 Gill T. The role of microfracture technique in the treatment of full-thickness chondral injuries. Oper Tech Sports Med 2000;8:138–140
4 Rodrigo JJ, Steadman JR, Silliman J. Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg 1994;7:109–116
5 Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 2001;391 (suppl): S362–S369
6 Steadman JR, Rodkey WG, Singleton SB. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop 1997;7:300–304
7 Blevins FT, Steadman JR, Rodrigo JJ, et al. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics 1998;21:761–768
8 Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg 1998;11:42–46
9 Steadman JR, Rodkey WG, Briggs KK, et al. The microfracture technique in the management of complete cartilage defects in the knee joint.[in German]. Orthopade 1999;28:26–32
10 Sledge SL. Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med 2001;20:365–377
11 Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg 1999;28:242–255
12 Norrdin RW, Kawcak CE, Capwell BA, et al. Calcified cartilage morphometry and its relation to subchondral bone remodeling in equine arthrosis. Bone 1999;24:109–114
< div class='tao-gold-member'>









