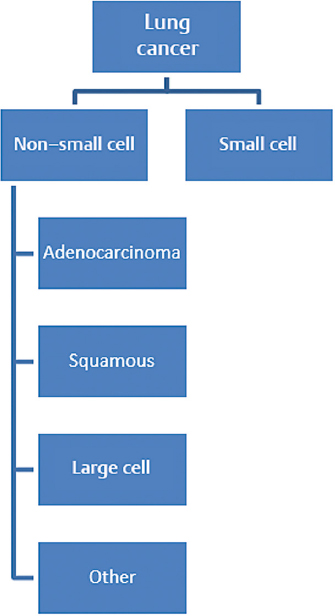
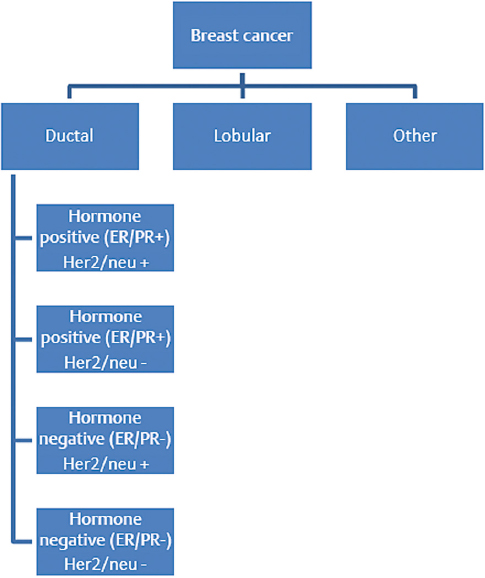
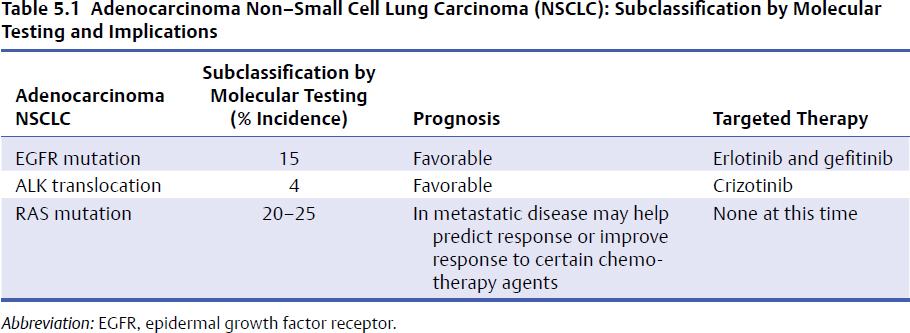
5 Primary tumors of the spine are rare and require a multidisciplinary approach to provide the patient with the best outcome. The members of the team bring their expertise and the underlying principles of their discipline. Medical oncologists have a solid understanding of the natural history and available systemic options for each tumor. Additionally, they are often able to develop a long-standing relationship with the patient and the family, thus providing the multidisciplinary team with insight on the goals and expectations of the patient, as well as their tolerance for treatment. This chapter discusses five principles of medical oncology that we consider key for all spine oncology surgeons when caring for patients with primary and metastatic tumors of the spine: 1. Tissue is the issue 2. Staging 3. The role of adjuvant therapy 4. Understanding the toxicity of therapy 5. Understanding the preferences of the patient These principles can be applied to the patient with localized or metastatic disease. This chapter also discusses common patient scenarios that illustrate and reinforce these principles. We do not cover each of the tumors in depth, as they are discussed in other chapters in this masters series. It is vital, when dealing with a patient with cancer, that we have the correct diagnosis. Obtaining appropriate tissue facilitates not only making the diagnosis but also performing additional testing on the tumor sample to help determine prognosis and treatment options. Advances in imaging, with present-day options including magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) scans, have enabled more accurate staging. Understanding both disease biology and imaging modality limitations is essential in guiding the choice of the appropriate imaging studies to properly stage patients. Adjuvant or neoadjuvant systemic therapy has improved the outcome of patients with primary bone tumors such as Ewing’s sarcoma and osteogenic sarcoma. The role of systemic therapy in the adjuvant setting with other primary spine tumors is less clear. However, there are circumstances where the use of adjuvant systemic options may positively impact the surgical approach. It is difficult to keep abreast of all the evolving therapeutic options and their associated side effects. These side effects, in particular, can have impact on the surgery and the postoperative course. The medical oncologist can be a valuable resource for information about these issues. Ultimately, achieving the patient’s goals is central to the care plan. As caregivers of patients with both curable and incurable cancers, we need to provide our patients with information about the potential benefits and side effects of any treatment we recommend, allowing them to be an active participant in the decision-making process. This first principle of medical oncology cannot be underestimated. Not only is tissue needed to make the diagnosis, but how we obtain the tissue and what additional testing can be done on that specimen has revolutionized the way we treat patients with cancer. In the past most of the biopsies that were needed to make the diagnosis were done with an open approach (open biopsy). The open procedure enabled the surgeon to obtain an adequate tissue sample to make the diagnosis and do any requisite ancillary testing. Additionally, the surgeon was able to control any bleeding that might have occurred during the biopsy procedure. The open biopsy often required the use of anesthesia and sutures. This added to the expense and recovery time. With the advancement of cross-sectional imaging, interventional radiologists are now able to perform percutaneous CT-guided biopsies on almost any tissue in any location. These tissue cores are for the most part enough to obtain a diagnosis and appropriate ancillary testing. This has reduced both recovery time and cost. The diagnostic accuracy for spine CT-guided biopsies can be has high as 97% with a low complication rate (see text box).1–3 Percutaneous CT–Guided Biopsy of the Spine: Accuracy in Diagnosis and Complication Rates Not only is tissue the issue to make the diagnosis, but it also provides tumor sample to perform additional ancillary testing to help determine prognosis and therapeutic options. Although these additional tests at present have limited value for primary bone tumors, they do have significant impact for nonprimary bone tumors for which spine oncologist are frequently consulted. We highlight two common tumors, lung cancer and breast cancer, for which additional ancillary testing is important in the management of the patient (Figs. 5.1 and 5.2). In the past, patients diagnosed with lung cancer were either classified as small cell lung carcinoma (SCLC) or non–small cell lung carcinoma (NSCLC). The NSCLC was further classified as squamous cell carcinoma, adenocarcinoma, large cell, or not otherwise specified (NOS). The lumping of all NSCLCs together despite the different histologies was largely due to the fact that our therapies were not that specific for the different histologies. With the increase in therapeutic options and improved subclassification of adenocarcinomas, we now know that all NSCLCs are not the same and their treatment and prognosis often differ. For instance, squamous cell carcinoma, which accounts for 20% of NSCLCs, occurs mainly in smokers, does not have mutations in the epidermal growth factor receptor, and is less responsive to the chemotherapy agent pemetrexed. When treated with a platinum-based doublet, patients with squamous histology who received cisplatin and pemetrexed had a 3-month decrease in their median overall survival compared to non–squamous cell histology, but had an almost 2-month improved median survival when they received cisplatin in combination with gemcitabine.4,5 Thus treatment decisions can be better tailored for patients with squamous cell carcinoma. For patients with adenocarcinoma NSCLC in addition to the histology, we now need to know the molecular characterization of the adenocarcinoma to better tailor treatment and provide prognosis (Table 5.1). The tissue samples of patients with adenocarcinoma NSCLC routinely undergo genotyping for epidermal growth factor receptor (EGFR) mutation, ALK (anaplastic lymphoma kinase) translocation, and Ras (rat sarcoma) mutations. The EGFR mutations occur in approximately 15% of adenocarcinoma NSCLC histology and generally occur in nonsmokers. These tumors are sen sitive to EGFR oral tyrosine kinase inhibitors such as erlotinib or gefitinib. and patients have a more favorable prognosis. The ALK translo cation similarly occurs in non-smokers, but in a smaller number of patients, occurring in less than 4% of patients with adenocarcinoma NSCLC. These tumors are sensitive to a new tyrosine kinase inhibitor, crizotinib, with improved prognosis. The KRAS mutation occurs in about 25% of adenocarcinoma NSCLC and is found in mainly smokers. The prognostic value of KRAS is limited, but therapeutic options and further studies are underway to determine if attacking the downstream target of KRAS might be helpful. Thus, in approaching patients with adenocarcinoma NSCLC, additional molecular testing can help with both prognosis and treatment options. Another common cancer that metastasizes to bone is breast carcinoma. The two main histological classifications of breast cancer are lobular and ductal. The vast majority of breast cancer is ductal (> 75%) and, just like NSCLC, can be further classified based on additional ancillary tumor testing. The three most common ancillary tests are estrogen receptor (ER), progesterone receptor (PR), and Her2Neu (HER2). Knowing the status of these three tests, the oncologist can help provide the patient with information about their prognosis and direct therapeutic options. Patients with tumors that are ER and PR positive are sensitive to hormonal therapy and have a significantly favorable prognosis compared with patients whose tumors are ER and PR negative. The HER2 oncogene is a member of the EGFR family and is also helpful in prognosis and tailoring treatment. Tumors that are HER2/neu positive are sensitive to drugs that target the HER2 receptor. Trastuzumab and pertuzumab are intravenously administered monoclonal antibodies that target HER2/neu, and lapatinib is orally administered and targets both EGFR and HER2. The addition of these drugs to standard treatment regimen improves the overall survival of patients with metastatic breast cancer. Patients whose tumors are negative for ER, PR, and HER2/neu are classified as having triple negative breast cancer and, in the metastatic setting, have decreased survival. Hence, tissue is the issue, having impact on the diagnosis, prognosis, and treatment decision making. Adequate tissue can be safely and accurately obtained by CT-guided biopsy to run the necessary ancillary tests. The second principle in medical oncology is staging—delineating the extent of the tumor. Historically, clinicians had to rely on physical examination findings or crude radiology imaging to get an idea of the exact location of the tumor and its extent within the body. Advances in radiology techniques have allowed us to stage the patient more accurately, but for certain tumors we still require additional testing. Moreover, we need to understand the pros and cons of each imaging modality. The appropriate staging tests depend on the type of tumor (tissue is the issue—the first principle of medical oncology) and understanding of the expected pattern of metastases. For the two most common primary solid bone tumors of the spine, Ewing’s sarcoma and osteogenic sarcoma, as well the common hematology primary bone tumors, lymphoma or multiple myeloma of the spine, staging studies differ (Table 5.2). Ewing’s sarcoma (or the Ewing family of tumors) is often evaluated initially with a plain X-ray of the affected bone. For suspicious lesions, CT imaging can help to determine the extent of cortical destruction, whereas MRI can better help to search for intramedullary disease (bone metastases) and soft tissue extension. Because Ewing’s sarcoma has a predilection to metastasize to the lung, a CT scan of the chest is an integral part of the staging process. Technetium bone scan of the entire skeleton completes the imaging evaluation for the presence of synchronous bone metastases. Although fluorodeoxyglucose (FDG)-PET is often used for staging, there are limitations and benefits compared with the CT chest and bone scan. Many of the FDG-PET/CT scans are low resolution, which decreases their ability to detect pulmonary metastases. In addition, routine FDG-PET/CT scans do not image all the bone in the body, and unless there is collaboration with the radiologist to cover all the bones, the patient might not be completely staging. FDG-PET imaging, however, is better than bone scans in detecting small bone lesions. Bone marrow biopsies (bilateral) have been historically performed for the staging of Ewing’s sarcoma because standard staging studies were not specific or sensitive enough to detect involvement of the bone marrow. The use of FDG-PET in addition to a diagnostic CT chest and screening MRI of the entire spine are becoming common staging studies for Ewing’s sarcoma. Additional blood testing, including a complete blood count (CBC), creatinine, and liver function tests, is also routinely done, and further evaluation would be needed if these tests are significantly abnormal. Table 5.2 Staging Studies Differences Between Ewing’s Sarcoma and Osteogenic Sarcoma
Medical Oncology Principles for the Spine Oncology Surgeon

 Introduction
Introduction
 Tissue Is the Issue
Tissue Is the Issue
 Accuracy
Accuracy
 71–97%
71–97%
 Complications
Complications
 0–21%
0–21%
 Pulmonary, neurologic, infectious, and bleed
Pulmonary, neurologic, infectious, and bleed
 Staging
Staging
| Staging Studies | Ewing’s Sarcoma | Osteogenic Sarcoma |
| CT | Chest | Chest |
| MRI | Of the affected lesion including the whole bone | Of the affected lesion including the whole bone |
| Technetium bone scan | Can be done, but FDG-PET/CT gaining favor | Preferred |
| FDG-PET/CT | Can be done if all bones are included and a diagnostic CT chest is done | Limited by lack of sensitivity to pulmonary metastasis and detection of small bone lesions |
| Bone marrow biopsy | Bilateral bone marrow | Not indicated |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; FDG-PET, fluorodeoxyglucose–positron emission tomography.
In contradistinction to Ewing’s sarcoma, osteogenic sarcoma rarely metastasizes to bone marrow, so bilateral bone marrow biopsies are not part of the routine staging studies. The CT chest and bone scan, as with Ewing’s sarcoma, are standard staging studies for osteogenic sarcoma given its predilection to metastasize to the lung and bone. The role of FDG-PET for osteogenic staging is limited by its lack of detection of small pulmonary metastases and less sensitivity in detecting bone metastases compared with technetium bone scans. The role of FDG-PET in more accurate assessment of response to preoperative therapy is evolving. Appropriate imaging of the local disease including the entire affected and adjacent bones with an MRI is part of the staging, as skip lesions can be detected.
The hematologic primary bone tumors lymphoma and multiple myeloma have different staging studies. Lymphoma can be classified broadly into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). Staging studies of HL include FDG-PET/CT, but bone marrow biopsies are unnecessary. For NHL, the standard staging is a CT of the chest, abdomen, and pelvis. In addition, because the bone marrow is involved 30 to 50% of the time, bilateral bone marrow biopsies are necessary to complete the staging. The use of FDG-PET is only for the aggressive histologies of NHL (tissue is the issue), such as diffuse large-cell NHL. Cerebrospinal fluid analysis is additionally included in the staging of central nervous system (CNS) lymphoma. Similar to Ewing’s sarcoma and osteogenic sarcoma, routine CBC, creatinine, and liver function tests are obtained, but serum protein electrophoresis (SPEP) and β2-microglobulin (indolent lymphoma) are also obtained for lymphoma.
Plasmacytoma/multiple myeloma is another common hematologic malignancy of the spine, and appropriate staging is helpful in guiding treatment options. Solitary plasmacytoma can occur in the bone, and staging is needed to exclude multiple myeloma. Staging studies include metastatic bone survey and FDG-PET or MRI of the entire spine and pelvis. In addition, laboratory studies, including CBC, creatinine, chemistry group, SPEP (serum protein electrophoresis), SIEP (serum immunolectrophoresis), quantitative immunoglobulins, urinary PEP (protein electrophoresis), and a unilateral bone marrow biopsy should be obtained. If the imaging studies do not show any additional lytic lesions and the criteria for multiple myeloma are not met on the lab tests or bone marrow biopsy, then treatment is primarily radiation. The benefits of additional systemic therapy are unclear, but up to 50% or more progress to multiple myeloma and 11% have local relapse, so long-term follow-up is needed.
Staging is essential in determining the extent of tumor involvement and ultimately choice of management. Each tumor varies in its predilection for the site of metastasis as well as sensitivity for detection on the varied imaging modalities. Thus the process of staging is tissue specific.
 Adjuvant Therapy
Adjuvant Therapy
The third medical oncology principle that all spine surgeons should know is the value of adjuvant therapy or neoadjuvant therapy. Adjuvant therapy is referred to as the additional therapy after the primary lesion has been completely removed. When therapy is used prior to resection of the tumor, this is referred to as neoadjuvant treatment. The main reasons oncologists use neoadjuvant/adjuvant therapy are to improve local control, preferably shrink/calcify the tumor to improve surgical morbidity, and to control any additional micrometastatic disease that might be present but not detected by conventional methods. The value of adjuvant/neoadjuvant therapy is most evident for primary tumors of the spine: Ewing’s sarcoma and osteogenic sarcoma. Ewing’s sarcoma is a systemic disease. Even though the tumor appears to be localized to one bone by appropriate staging studies, without chemotherapy, the risk of recurrent and metastatic disease is over 80%.6 With the addition of adjuvant/neoadjuvant systemic chemotherapy, the cure rate can approach 80%. Osteogenic sarcoma has a similar 80% rate of metastatic disease without adjuvant/neoadjuvant systemic therapy. With the addition of chemotherapy, the cure rates are around 60 to 70%.7
The selection of adjuvant versus neoadjuvant chemotherapy is often based on historical data, but there are additional reasons to select one approach over the other. In principle, the use of neoadjuvant therapy will enable a reduction in tumor size, thus potentially facilitating surgical resection with appropriate margins. This can be especially valuable for large tumors that are “borderline resectable” or where critical neurologic or vascular structures can be spared. Neoadjuvant therapy also enables the natural biology of the cancer to declare itself. This is especially helpful when the oncology team and patient are contemplating an aggressive life-changing surgery, and additional time prior to surgery is needed to see if metastatic disease will develop and obviate the need for a life-changing curative surgery. In this situation, neoadjuvant chemotherapy can be used to help determine if the tumor is responsive to chemotherapy and the likelihood of controlling the micro-metastatic disease. Another benefit of neoadjuvant chemotherapy is its prognostic value in estimating the percent of necrosis after chemotherapy (pathological response). For osteogenic sarcoma, after two to four cycles of neoadjuvant chemotherapy, tumors at the time of resection that have ≥ 90% necrosis have a favorable outcome compared with those with < 90% necrosis.8,9Whether adjuvant intensified chemotherapy for tumors with < 90% necrosis improves overall survival is not known, and the results of the completed EURAMOS study are eagerly awaited.10
There are times when neoadjuvant therapy, although preferred, is not administered. Such incidental discoveries of malignancy can occur with excisions of presumed benign tumors, or removal of lesions found during repairs of fractures. In these situations, there is evidence for both Ewing’s sarcoma and osteogenic sarcoma that when all the normally planned chemotherapy is given postoperatively, overall survival is not compromised.11,12 Another scenario where neoadjuvant therapy is withheld is in patients whose clinical status is insufficient to withstand or tolerate systemic chemotherapy until the primary tumor is removed. The one caveat is that patients with Ewing’s sarcoma can have significant systemic symptoms and appear not to be chemotherapy candidates, but because Ewing’s sarcoma is very sensitive to chemotherapy, these patients can have dramatic clinical response, so all efforts to administer neoadjuvant chemotherapy should be tried. Other reasons to go directly to surgery without neoadjuvant chemotherapy are if the tumor is causing significant clinical compromise, or if a minor increase in the tumor size could be catastrophic, precluding curative resection. In these situations, a solid understanding of the likelihood of shrinkage of the tumor with neoadjuvant chemotherapy is needed to justify delaying surgery. For instance, primary osteogenic sarcoma of the spine historically does not shrink with neoadjuvant chemotherapy. On the other hand, Ewing’s sarcoma has a greater chance of responding both in tumor size and percent necrosis and thus justify neoadjuvant chemotherapy, with a delay in surgery. As with all these situations, active collaboration between the members of the multidisciplinary team is critical in helping to make the best decision for the patient.
Adjuvant therapy is also routinely given after neoadjuvant chemotherapy and surgery to continue to control any additional micrometastatic disease. As noted above, intensification of adjuvant therapy for poor responders (< 90% necrosis) for osteogenic sarcoma is not known to impact survival, but additional therapy should be administered. Significant delay in resumptions of systemic chemotherapy on an adjuvant basis can compromise overall survival. Good communication and coordination with the medical oncologist for timely resumption of chemotherapy is important when the patient has appropriately recovered.
Unfortunately, not all patients are able or willing to resume chemotherapy after surgery. Determining this prior to surgery could help guide the surgeon on how close the margins can be or how much function should be compromised if overall survival will be reduced without adjuvant chemotherapy. Although by definition it is not considered adjuvant therapy if given when gross disease remains or there are positive resection margins (R2 and R1 resection, respectively), knowing the histology of the tumor could enable appropriate R2 or R1 resection followed by postoperative therapy, especially if the tumor is sensitive to both chemotherapy and radiation. The dilemma is that the oncology team cannot always predict who will be unable to undergo adjuvant therapy or postoperative therapy.
For the primary bone tumors of the spine (Ewing’s sarcoma and osteogenic sarcoma), the main approach is to administer both neoadjuvant and adjuvant systemic therapy with R0 resection when possible.
 Toxicity of Systemic Therapy
Toxicity of Systemic Therapy
The fourth medical oncology principle is to understand the toxicities of therapy. The importance of this principle for the oncological spine surgeon is in determining the optimal timing for surgery in relation to chemotherapy and to appropriately manage the patient in the postoperative period. The vast majority of chemotherapy drugs used to treat Ewing’s sarcoma and osteogenic sarcoma of the spine and bone are cytotoxic agents that can affect the bone marrow as well as the kidney and liver. Most of the toxicity is evident by the low counts for CBC, elevated creatinine for the kidney, and elevated liver function tests for the liver. One has to be aware that despite normal counts at the time of chemotherapy administration, there is a known nadir period for these drugs. Recovery can be as quick as 2 weeks but can be delayed due to cumulative toxicity. Some of these drugs can affect the kidney’s ability to maintain electrolyte balance (Fanconi’s syndrome) despite a normal creatinine.
For metastatic disease from other solid tumors, there are many new agents that target the vascular endothelial growth factor and predispose patients to bleed and clot. The agent bevacizumab should be stopped up to 4 weeks prior to elective surgery to reduce the risk of bleeding.13 In some patients there is no time to delay surgery, and managing the bleeding will have to be done expectantly. Coordination and collaboration with the treating medical oncologist to select an appropriate time for surgery is ideal. There are many new orally administered tyrosine kinase inhibitors to vascular endothelial growth factor (VEGF) that have significantly short half-lives compared with bevacizumab, and most clinical trials recommend that these agents be stopped 1 week prior to surgery, but there are immerging data that some of these drugs can be stopped as short as 1 day prior to surgery.14 The complication rate after discontinuation of a VEGF inhibitor is likely also related to the location and histology of the tumor, and continued caution should be practiced when operating after stopping a VEGF inhibitor.
Certain drug exposures are important in managing the perioperative period. The best known is the prior exposure of the patient to bleomycin. This drug accumulates in the lung and can cause lung damage when a high concentration of oxygen is used perioperatively. The newer targeted agents have a host of adverse events that are not typically seen with standard cytotoxic agents. These adverse events should be noted and managed accordingly (see text box).15
Common Adverse Events with Agents that Target VEGF
 Hypertension
Hypertension
 Thrombotic risk
Thrombotic risk
 Hemorrhage
Hemorrhage
 Wound complications including fistulas
Wound complications including fistulas
 Reversible posterior leukoencephalopathy syndrome
Reversible posterior leukoencephalopathy syndrome
 Cardiomyopathy
Cardiomyopathy
 Proteinuria
Proteinuria