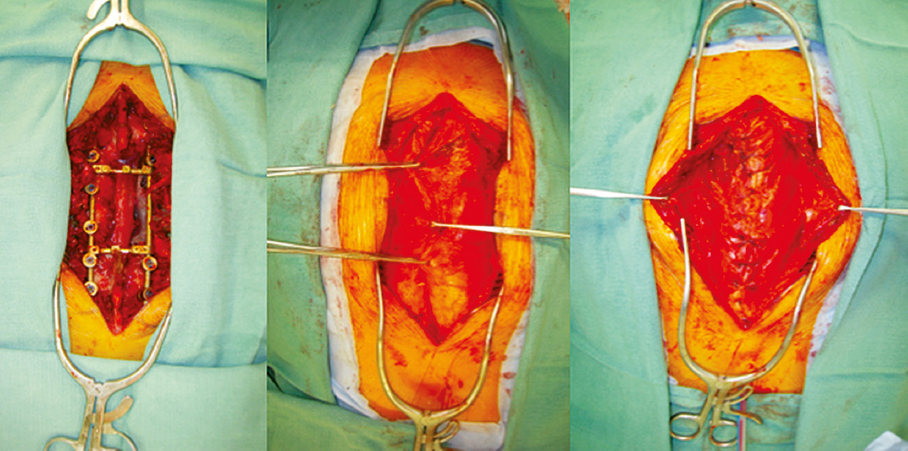
15 Radical oncological resection is warranted for aggressive benign tumors and for low-grade and high-grade malignant tumors of the spine. Advances in neurosurgical technique, instrumentation for spinal stabilization, and adjuvant therapy allow for a greater number of surgical candidates than ever before.1 However, surgical wounds that contain hardware and vital neural structures can have devastating consequences for patients if the wounds become exposed or infected. Complex, composite wounds of the spine are managed with immediate soft tissue reconstruction using muscle or fascial flaps.2 This is particularly indicated for those patients identified as being at high risk for wound-healing complications, such as those with prior operations, infections, potential for planned placement of spinal instrumentation, or medical comorbidities that predispose to complications.3 The oncological spine surgeon should work in tandem with the plastic and reconstructive surgeon to optimize both the ablative and the reconstructive surgical procedure. Reconstructive options range from primary closure to local muscle flaps to more complex methods of reconstruction such as microvascular free tissue transfer. Defects in regions that have been exposed to prior irradiation or surgery, or have a paucity of local tissue require more complex types of reconstructive interventions. Using a combination of these techniques, the goal of the reconstructive surgeon, in tandem with the neurosurgical team, is to obliterate dead space and provide coverage of vascularized soft tissue over critical areas in an effort to prevent infection and restore function to these patients. The physiological status of the patient must be considered and balanced with the overall reconstructive plan. The overall prognosis of the patient must also be taken into account as well. Reconstruction to improve a patient’s quality of life is often considered even in a palliative scenario.4 The patient’s medical history should be thoroughly reviewed to stratify the relative risk of infection. Evidence of malnutrition, smoking, diabetes mellitus, and peripheral vascular disease should be evaluated, as these conditions can have deleterious effects on wound healing as well as flap survival.5 Radiation therapy induces tissue injury through changes in the microcirculation of the defect and its surrounding areas, leading to decreased perfusion and impaired wound healing. Indeed, spinal surgery patients who undergo adjuvant radiation have up to three times the rate of wound healing complications as compared with their nonradiated counterparts.6 For this reason, the reconstructive surgeon should be cognizant of the timing, dosage, and location of any prior or planned radiation. Additionally, the plastic and reconstructive surgeon should utilize tissue outside the field of radiation for flap reconstruction. This will take the form of a pedicled or free tissue transfer. A variable amount of skin, adipose, fascia, muscle, and bone can be rotated or transplanted on a vascular pedicle composed of a specific artery and vein. The timing of spinal soft and hard tissue reconstruction is most often dictated by the status of the tumor and the surgical margins. Certain patient factors such as advanced age, multiple comorbidities, or the need for locoregional control with adjuvant radiotherapy must also be considered in the timing. Primary reconstruction carries a significantly decreased rate of wound healing complications and is preferred to delayed reconstruction.7 Delayed reconstruction, although not optimal, is occasionally unavoidable for defects with extensive soft tissue deficits or in cases of patient instability. If a patient requires delayed reconstruction, a negative pressure closure device is the preferred temporizing measure until definitive reconstruction can be performed at a later date. Negative pressure closure devices over exposed neural structures in the region of the spinal cord can create both cerebrospinal fluid leaks and pain. Preoperative imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI) should be performed to evaluate the integrity of the surrounding soft tissue, vascular anatomy, and any previously placed hardware. Often, local tissue, such as the paraspinal musculature, will be sought to obliterate surgical defects; however, if this tissue is not available, the reconstructive surgeon will have to plan in advance to utilize regional or distant tissue outside of the zone of injury to reconstruct the defect. Knowledge of the associated vasculature helps with preoperative planning and with the eventual intraoperative decisions. The core principle underlying reconstructive algorithms used by plastic surgeons is to progress from simple to more complex reconstructions on the basis of the specific wound requirements. The underlying goal for the reconstruction is to close a wound primarily with local tissue that will be tension-free and obliterate dead space. When primary closure is not feasible, local tissue flaps of tissue can be used. Local tissue flaps enable surgeons to reconstruct soft tissue defects with similar tissue from an adjacent location. “Random” local flaps comprise adjacent skin and subcutaneous tissue and are based on a subdermal plexus vascular supply. By definition, a random flap does not have a distinct, specific blood supply. In contrast, axial-pattern flaps are based on specific blood vessels. Axial flaps can be fasciocutaneous (deep muscle fascia with overlying skin), myocutaneous (muscle with skin), or osteocutaneous (bone with overlying skin); these flaps enable reconstructive surgeons to repair defects with tissue that is similar to the resected tissue. Microvascular free tissue transfer involves harvesting a tissue construct and its named blood supply from a distant region of the body and placing it into a defect. Vascular anastomosis between the flap’s donor vessels and the patient’s recipient vessels is performed under magnification provided by a surgical microscope. The decision to use a particular flap is based on the requirements for replacing missing skin, adipose tissue, fascia, muscle, or bone. The primary advantage of microvascular free tissue transfer is that tissue of a quality similar to that of the resected tissue can be moved from a remote part of the body, thereby enabling optimal aesthetic and functional outcomes. This also enables irradiated or infected tissue to be removed and replaced with soft, pliable, and vascularized tissue. The drawbacks of free tissue transfer are related to donor-site morbidity and the potential for longer operative times. However, with careful coordination, harvests of vascularized osteocutaneous flaps (e.g., free fibula flap) can be performed simultaneously or in concert with oncological resections. Spinal soft tissue defects can be classified according to a scheme suggested by Casas and Lewis8 in which wounds are divided into the upper third, middle third, and lower third. The upper third of the spine includes all cervical vertebrae up to T7. The middle third of the spine includes T1 to T12. The lower third of the spine includes vertebrae L1 to S5.8 Defects of the upper third of the spine, defined by the spinous processes of C3 through C7, are among the most commonly encountered by the plastic surgeon and are amendable to a multitude of axial pattern flaps. The majority of these defects can be reconstructed using bilateral paraspinal flaps. Additionally, a unilateral trapezius muscle flap can be used when the paraspinal muscles have been compromised. A latissimus dorsi muscle with or without a skin paddle can be harvested for larger, more extensive defects.8 The middle third of the spine is delineated by the spinous processes of C7 to L1. Reconstruction of this area is similar to that of the upper third, where bilateral paraspinous muscle flaps are able to provide coverage for a majority of defects. If these muscle flaps are unavailable due to trauma, irradiation, or defect size, the latissimus dorsi muscle can provide durable coverage either as a standard, axial pattern flap or by utilizing reverse latissimus muscle flap.9 The boundaries of the lower third of the spine are dictated by the spinous processes of L1 to S5. Due to the proximity of this region to the gluteal and posterior thigh regions, there is a multitude of potential flap donor sites available for flap reconstruction. Within this region, the paraspinous muscles are robust and readily available for coverage of most defects. If they are unavailable, other regional flap options are the latissimus dorsi turnover muscle flap, gluteus muscle flap, and pedicled vertical rectus abdominis myocutaneous flap (VRAM) based on the deep inferior epigastric vessels. When utilized in the lower third, the pedicled VRAM is routinely used to obliterate the actual space created by a subtotal or total sacrectomy. In these clinical situations, the bowel can herniate posteriorly and become entangled in spinal hardware or develop a bowel obstruction. In the setting of a total sacrectomy, the free fibula bone can be anastomosed to the superior blood supply of a pedicled VRAM or through a vein graft to the intercostal vessels.10 Negative pressure-assisted closure can provide for temporary coverage in soft tissue spinal defects when definitive reconstruction is delayed. When utilized appropriately, this device can promote neovascularization, decrease edema, and increase local granulation tissue, as well as provide contractile force at wound edges.11 Although this modality can be used to prepare the wound bed for definitive reconstruction with soft tissue flaps in a delayed fashion, it can also be used to promote healing by secondary intention in partial-thickness defects. Tissue expansion is a process in which an inflatable prosthetic implant with a silicone shell is used to expand local and regional tissues so that they can eventually be advanced into the wound in a delayed fashion. The inflatable implant is inserted at the time of tumor extirpation or during a second procedure. At subsequent office visits, saline is injected through an integrated or remote port to gradually expand the implant. Once the tissue has been sufficiently expanded, it can be advanced into the defect. Because tissue expansion takes time, the method is not feasible for spinal reconstruction that requires immediate coverage of spinal instrumentation or neurovascular structures. Risks of tissue expansion include infection, extrusion, and rupture of the implant.12 A recently adapted alternative to internal tissue expansion is external tissue expansion. This technique relies on the application of a constant, external force to the underlying skin, fascia, and muscle. There are several adapted means of expansion using different tensioning devices that are often secured to the periphery of the wound. As the external forces are applied to the overlying skin and fascia, several cytoskeletal and extracellular changes occur to produce a biochemical response that results in an overall increase of tissue mass and size.13,14 Examples of external expander systems include Jacob’s ladder, retention sutures, and commercially available external expansion systems. Each of these techniques applies external force and tension to the edge of the defect to produce a gradual closure using autologous tissue. The disadvantages of this technique include the lengthy time required for closure as well as pain and discomfort during the expansion process. These are not primary or secondary options in spinal soft tissue reconstruction but must be considered in the clinical setting of the patient. Commercially available biological tissue matrices (BTMs) currently come from five different sources: human dermis, porcine dermis, porcine small intestinal submucosa, bovine dermis, and bovine pericardium.15 These BTMs can be used to reconstruct soft tissue defects where there is a paucity of available donor tissue. Although each BTM is inherently different due to its proprietary processing technique, the central tenet that all BTMs should be based on is the provision of strength and early revascularization capacity in an effort to provide soft tissue coverage and resist infection. Within spinal and sacral reconstruction, BTMs can be used in a multitude of capacities including the provision of durable coverage over implanted hardware as well as the creation of pelvic diaphragms to prevent visceral herniation into low sacral defects.16 These defects, as mentioned previously, arise from subtotal or total sacrectomy defects. The paraspinous muscles are a multilayered group of muscles that line each side of the spinal column and have the potential to provide excellent coverage of moderately sized defects at almost any level of the spinal column. Although composed of nine paired muscle groups, the collective flap is referred to as the paraspinous muscle flap and is transposed based on either the medial or lateral perforating intercostal vessels. The superficial layer is composed of the splenius capitis and the splenius cervicis muscles, both of which are found in the cervical spine, or upper third. The intermediate layer of the spinal column is composed of the iliocostalis, the longissimus, and the spinalis muscles, and these span the entire length of the spinal column. Finally, the deepest layer includes the transversospinal, semispinalis, multifidus, and rotatores muscles. These paired midline muscles are dissected superficially to reveal the extent of the lateral border. Then the dissection proceeds along the medial undersurface of the muscle groups, and the paraspinous muscles are elevated off of the ribs, with care taken to preserve the lateral intercostal perforators. After elevation, the paraspinous muscles are manually advanced toward the midline and sutured together.17 The advantages of the paraspinous muscle flaps include convenience as well as a relative ease of dissection. Additionally, there is often no donor-site defect, as many times the flap is within the wound bed. However, because of this proximity to the wound, the flap is often compromised secondary to resection and is not available for reconstruction (Fig. 15.1). The trapezius flap can be harvested as a myocutaneous or muscle-only flap and was first described in 1979.18 The flap is based off of the descending branch of the transverse cervical artery and vein. In addition to the dominant blood supply from the transverse cervical system, it also receives minor contributions from the dorsal scapular artery and perforating posterior intercostal vessels. This flap is useful for the coverage of defects in the upper third, or cervical region of the spinal column. The advantages of this flap include a relatively flat and thin cutaneous portion that can be useful in shallow defects. The disadvantages include a relatively limited arc of rotation and significant donor-site morbidity of upper extremity weakness. Additionally, due to the proximity of the muscle to the operative field, the muscle may be damaged or irradiated, leading to inability to use the flap (Fig. 15.2). The latissimus dorsi muscle flap is a versatile flap and can be harvested either as a muscle only or as a myocutaneous flap. This flap can be used to reconstruct upper, middle, and lower areas of the spinal column and has a remarkable arc of rotation.19 The latissimus dorsi muscle has a dual blood supply. The dominant vascular pedicle is derived from the thoracodorsal artery, and the flap also receives a significant supply from the segmental intercostal vessels that enter the muscle at the midline.
Wound Closure Techniques

 Introduction
Introduction
 Preoperative Evaluation
Preoperative Evaluation
Medical Comorbidities
Radiation Therapy
Timing of Reconstruction
Imaging
 Reconstructive Surgical Tenets
Reconstructive Surgical Tenets
 Anatomic Location and Axial Pattern Flap Availability
Anatomic Location and Axial Pattern Flap Availability
Upper Third
Middle Third
Lower Third
 Adjuncts to Flap Surgery
Adjuncts to Flap Surgery
Negative Pressure Wound Therapy
Internal Tissue Expansion
External Tissue Expansion
Biological Tissue Matrices
 Characterization of Axial Pattern Flaps
Characterization of Axial Pattern Flaps
Paraspinous Muscle
Trapezius Muscle
Latissimus Dorsi Muscle
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree