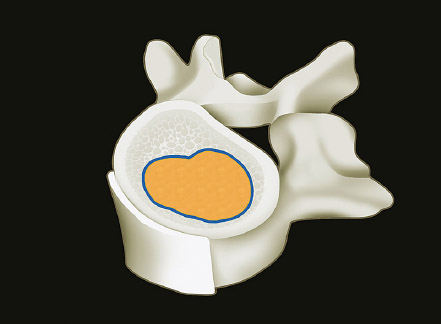
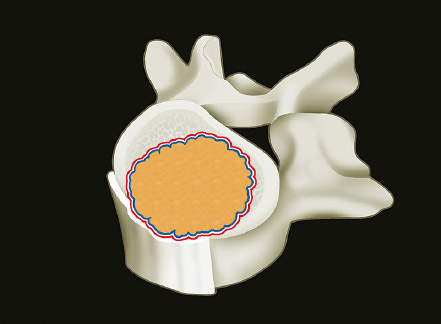
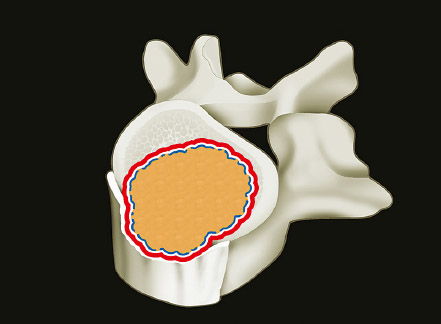
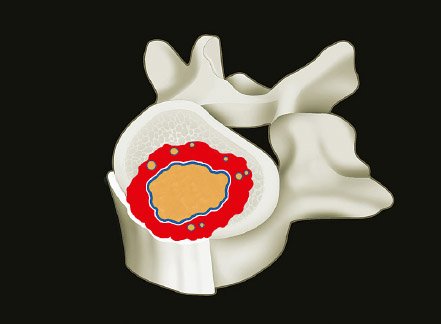
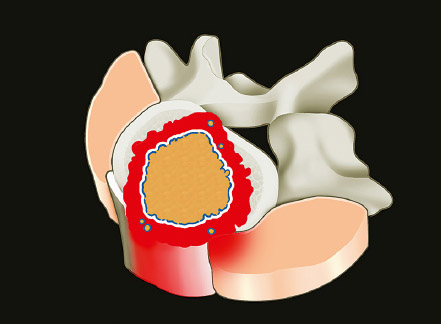
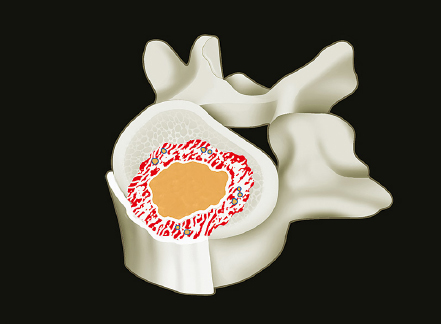
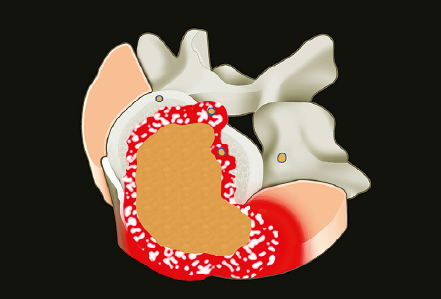
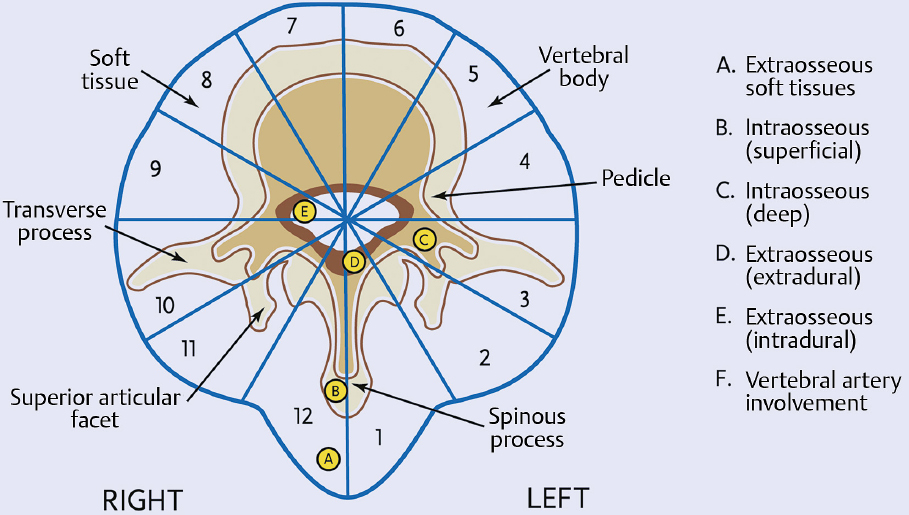
1 Primary bone tumors of the spine are very rare,1,2 comprising only 10% or less of all bone tumors. In the United States about 7,500 new cases are estimated per year. The overall world occurrence can be expected to be 2.5 to 8.5 cases per million inhabitants per year. Compared with primary spinal tumors, metastatic tumors are much more common; the spine is the most common skeletal region for secondary tumors. The occurrence of spinal metastases in the clinical course of common solid tumors is reported to be 20 to 70%; therefore, a 30 to 50 times more frequent incidence is reported compared with that of primary bone tumors of the spine. Of 43,735 primary bone tumors reviewed in the literature on bone tumors, 1,851 (4.2%) were located in the spine above the sacrum. These figures explain why they are often unsuspected, misdiagnosed, and, in many cases, unfortunately, incorrectly treated. As the occurrence of lumbar stenosis, cervical radiculopathy, and acute spinal cord injury is estimated at 300 cases, 83 cases, and 5 cases per 100,000 persons per year, respectively,2 an expertise in diagnosis and treatment strategy is required for early recognition of a spine tumor and for differentiating a primary tumor from a metastatic tumor. Back and neck pain related to activity are very frequent symptoms, particularly in adults, and mostly related to disk prolapse, degenerative changes, and myofascial strains. Conversely a spine tumor should be first suspected when a patient with a history of cancer (current, recent, or in the past) complains of back or neck pain, particularly if it is progressive, unrelenting, not closely related to activity, and increasing during the night. Tumor growth may cause expansion of the bony cortex of the vertebral body, which results in pathological fracture and invasion of paravertebral soft tissues. Pain in these cases can be associated with acute or chronic compression of neurologic structures that can result in radiculopathy and or myelopathy. Some lesions do not cause pain. Latent lesions (hemangiomas, fibrous dysplasia, exostosis) are asymptomatic by definition, and diagnosis is achieved incidentally on imaging performed for other reasons. Cases of incidental detection of chordoma are also reported. This malignant tumor is characterized by very slow growth and, in anatomically voluminous regions such as the sacrum, can grow to very large sizes before detected. Painful scoliosis in an adolescent is strongly suggestive of osteoid osteoma or osteoblastoma. Usually not visible on standard radiograms, the fixed curve without a compensatory curve and rotation is strongly suggestive. Technetium isotope bone scan helps to localize the lesion. A computed tomography (CT) scan, performed at the level of intense uptake, displays the pathognomonic image of osteoid osteoma: a small island of pathological ossification surrounded by a lytic halo and frequently by a wide rim of reactive bone formation. Technetium isotope bone scan is also able to localize multiple lesions. The role of positron emission tomography (PET) scan is becoming more relevant, particularly in its ability to differentiate tumors from infectious diseases. Imaging studies (CT scan and magnetic resonance imaging [MRI]) can generate a working diagnosis, as some patterns are quite characteristic. Giant cell tumor and Ewing’s sarcoma are lytic conditions, and most osteosarcomas are characterized by extensile aggressive pathological bone formation with ill-defined borders. A multicameral balloon-like pattern with double-density content is typical of aneurysmal bone cysts. Infiltrating erosions inside the cancellous bone of a vertebral body arising from the posterior wall is suggestive of chordoma. Soft tissue masses arising from the posterior elements with rounded calcifications are typical of peripheral chondrosarcomas. Angiography shows pathological vascularity, and selective arterial embolization has become an indispensable tool to reduce intraoperative bleeding. Histological diagnosis should always be achieved by biopsy, but clinical, laboratory, and imaging studies are important to orientate diagnosis and to select the biopsy technique. The goal of a biopsy is to obtain a specimen of the tumor, which is representative of the lesion and large enough to allow histological and ultrastructural analysis as well as immunologic stains. The surgeon must recognize the vital part of the tumor and discard the necrotic or reactive part. Cultures should be taken to rule out infection. There are three traditional biopsy techniques: incisional, needle or trocar, and excisional. A number of basic principles should be observed when performing incisional and excisional biopsies in order to prevent tumor contamination of the surrounding tissue, which is the major risk of biopsy. Transverse incisions and flaps should be avoided; the tumor should be approached in the most direct manner possible, avoiding the anatomic interspaces (so-called extracompartmental spaces) commonly followed in orthopedic surgery. The approach should cross muscular structures. Tissues should be handled carefully, hemostasis should be meticulous, and suturing performed in all the anatomic layers. Bleeding from exposed bone or from uncauterized vessels and injured muscle will form a postoperative hematoma that may carry tumor cells beyond the margins of the intended excision and contaminate tissues proximal or distal to the primary lesion. The margin of the soft tissue component of the mass is often the most helpful to biopsy, as central portions are frequently necrotic. The surgeon should take care not to crush or distort the specimen, so as to maintain its architecture. Finally, if the definitive excision follows the biopsy during the same session, it is essential that all instruments used during the biopsy be discarded. The field should be re-draped, and the surgeons should change gowns and gloves before the definitive excision. If fusion is planned, bone graft should be taken through a separate surgical setup. If the planned definitive procedure is an en-bloc resection, the biopsy incision from the skin to the tumor mass should be placed so that it may be excised in a single block with the tumor and its margins. This is rarely possible in the treatment of tumors of the spine, if the epidural space has been violated. Frozen section biopsy can be obtained during the excisional procedure when the diagnosis is highly probable and just requires confirmation. In rare situations, a patient may have two separate primaries; if there is any doubt, the results of the frozen section should be received before proceeding with the resection, such as in a situation of an assumed spine metastasis. Excisional biopsy can be considered only for conditions whose radiographic pattern is pathognomonic, for example osteoid osteoma. The correct way to obtain a tumor sample from a vertebral body without violating the epidural space is with a transpedicular biopsy. The pedicle must be perforated by a drill or trocar. The surgeon must be careful not to perforate the pedicle wall, causing epidural space contamination. A small straight curette is then introduced to remove the specimen. The perforated pedicle must be filled with acrylic cement to prevent tumor backflow. The soft tissue track can be removed together with the surrounding muscle. Small trocar biopsies are subject to sampling errors and provide small specimens for evaluation. The authors’ preferred technique is to biopsy with a 12-gauge trocar performed under CT guidance. This technique produces tissue cores that allow for histological architecture and immunohistochemical studies. CT guidance allows the interventional radiologist to reach even the smallest lesions inside the vertebral body. Diagnosis should always be confirmed on histological diagnosis; biopsy is mandatory. Conversely, some latent, asymptomatic lesions such as hemangioma, fibrous dysplasia, and exostosis are characterized by such a pathognomonic imaging that a biopsy is not always necessary. When this is the case it is recommended to follow the evolution of the disease by sequential imaging, initially every 4 to 6 months, to fully assess the latency of the condition. In rare situations a patient may present with neurologic deterioration in a setting of a possible primary tumor. The surgeon is faced with the decision to stage the tumor and obtain a diagnosis or proceed with neurologic decompression, potentially rendering the patient incurable due to the tumor being violated. Each case is different, and conditional probabilities must be considered and integrated in a shared decision-making process with the patient. Often some delay in the treatment can be achieved with the use of steroids. Enneking et al3 proposed in 1980 a system to stage the biological behavior of tumors of bone and soft tissue. This system was later applied also to the spine in several reviews. This system divides benign tumors into three stages (S1, S2, and S3) and localized malignant tumors into four stages (IA, IB, IIA, and IIB). Two further stages include metastatic high-grade intra- and extracompartmental malignant tumors (IIIA and IIIB). This classification is based on clinical, imaging, and histological findings. Each stage is related to overall prognosis and, by necessity, directly linked to categories of surgical procedures based on the concept of “margin.”3,4 The first stage of benign tumors (S1, latent, inactive; Fig. 1.1) includes asymptomatic lesions, bordered by a true capsule, which is usually seen as a sclerotic rim on plain radiograms. These tumors do not grow, or grow very slowly. No treatment is required, unless surgery is needed for decompression or stabilization. Benign stage 2 tumors (S2, active; Fig. 1.2) grow slowly, causing mild symptoms. The tumor is bordered by a thin capsule and by a layer of reactive tissue, sometimes found on plain radiograms as an enlargement of the tumor outline. The bone scan is positive. An intralesional excision can usually be performed with a low rate of recurrence. Based on very low quality evidence, the incidence of recurrences can be lowered further by local adjuvants (cryotherapy, embolization, radiation therapy). Benign stage 3 tumors (S3, aggressive; Fig. 1.3) are rapidly growing, with a capsule that is very thin, discontinued, or absent. The tumor invades neighboring compartments, and a wide reactive hypervascularized tissue (pseudocapsule) is often found, sometimes permeated by neoplastic digitations. The bone scan is highly positive. Indistinct margins are seen on plain X-rays, CT scan shows extracompartmental extension, and MRI clearly defines a pseudocapsule with significant edema and reaction. Intralesional excision (curettage), even if augmented by radiation, can be associated with a significant rate of local recurrence. En-bloc excision with marginal or wide margins is the treatment of choice. Fig. 1.1 Stage 1 benign tumor. The tumor is latent and inactive, and a capsule surrounds the lesion. Fig. 1.2 Stage 2 benign tumor. The tumor grows slowly. The capsule is thinner. The tumor is surrounded by reactive tissue, as expression of local reaction to tumor growth. Fig. 1.3 Stage 3 benign tumor. The tumor grows faster and invades early the epidural space or the perivertebral region. The capsule is very thin, and the reactive zone is wider. Fig. 1.4 Stage IA malignant tumor: low grade, intracompartmental. The pseudocapsule may include a small island of tumor. Fig. 1.5 Stage IB malignant tumor: low grade, extracompartmental. The pseudocapsule may include a small island of tumor. Low-grade malignant tumors are assigned stage I, subdivided into IA (the tumor remains inside the vertebra; Fig. 1.4) and IB (tumor invades paravertebral compartments; Fig. 1.5). No true capsule is associated with these lesions, but a thick pseudocapsule of reactive tissue permeated by small microscopic islands of tumor is seen. A resection performed along the pseudocapsule (marginal) can leave residual foci of active tumor; stereotactic radiation or proton-beam therapy can be added to reduce the risk of recurrence. The treatment of choice, if feasible, is a wide en-bloc resection, and careful marginal dissection if neurologic tissues are involved. High-grade malignancies are defined as IIA (Fig. 1.6) and IIB (Fig. 1.7). The neoplastic growth is so rapid that the host has no time to form a continuous reactive tissue. There is continuous seeding with neoplastic nodules (satellites). Moreover, these tumors can have neoplastic nodules at some distance from the main tumor mass (skip metastases). These malignancies are generally seen on plain radiograms as radiolucent and destructive and in many cases are associated with a pathological fracture. Invasion of the epidural space is rapid in stage B, particularly in small cell tumors (Ewing’s sarcoma, lymphomas) characterized by semifluid tissue, which are able to occupy the epidural space after infiltrating the cortical border of the vertebra. Fig. 1.6 Stage IIA malignant tumor: high grade, intracompartmental. The pseudocapsule is fully invaded by the tumor. Fig. 1.7 Stage IIB malignant tumor: high grade, extracompartmental. The pseudocapsule is fully invaded by the tumor. A small island of tumor can be found outside the main tumor mass. The margin of the resection must be wide (it is not possible to achieve a “radical” margin, in the spine), and courses of radiation and chemotherapy (according to the tumor type) must be considered for the local control and for the avoidance of distant spread. Often in areas of anatomic constraints, marginal margins (discussed below) or planned intralesional transgressions must be accepted, or consideration given to the sacrifice of neurologic structures. Stages IIIA and IIIB describe the same lesions as IIA and IIB, but are associated with distant metastasis, and in the majority of situations would be considered incurable. As staging must be based on the natural history, surgical planning must be based on what the procedure achieves in relation to the margin of the lesion. The final pathological margin achieved through surgical technique is directly related to prognosis. Stener and Johnsen5 were the first (to our knowledge) to apply extremity oncological principles to the spine; these concepts were published in exhaustive reports of en-bloc resections in the spine. Roy-Camille and Tomita later popularized the techniques of en-bloc resection. Many papers reviewed series of patients and provided tips and details for various techniques.1,6–9 Since the late 1980s Weinstein and Boriani10 tried to focus on a unified classification that proposed envelopes of resection as Enneking et al3 had done for the extremities. By retrospectively reviewing his large case series, Boriani was able to establish significant relationships between margin and outcome specific to the spine.
Evaluation and Decision Making

 Introduction
Introduction
 Clinical Findings and Imaging
Clinical Findings and Imaging
 Biopsy
Biopsy
 Oncological Staging
Oncological Staging
Benign Tumors
Malignant Tumors
 Margins and Surgical Planning
Margins and Surgical Planning
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree