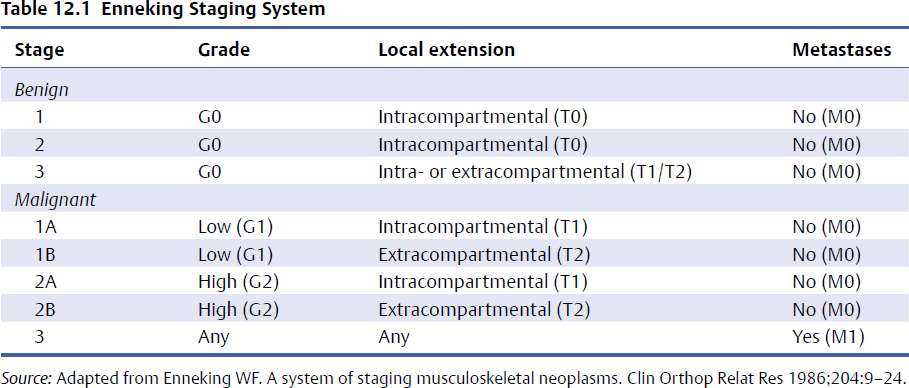
12 Primary tumors of the spine are rare. As a result, clinical expertise has been concentrated in selected quaternary referral centers. In recent years these centers have collaborated and combined the best available evidence with their collective expert opinion to enhance the published literature and further define the essentials of successful surgical care. Surgery plays a key role in the management of most primary spinal tumors. Historically, inferior results and high recurrence rates were reflective of a poor understanding of the fundamentals integral to the successful operative management of these rare entities, coupled with the technical complexities of their surgical approach. More recently, technological advances and technical descriptions of a number of surgical approaches have demonstrated the feasibility of following evidence-based oncological care principles. Despite this, and because of the potential for severe and unacceptable neurologic deficits and the intrinsic stability function of the spinal column, surgery remains challenging. This chapter discusses the key principles to consider when selecting a surgical approach and illustrates them with representative cases. Originally described in the 1980s for primary tumors of the appendicular skeleton, the Enneking classification remains the fundamental foundation for approaching primary mesenchymal tumors, including those originating from the spine.1 This system classifies primary tumors based on three factors: a grade of their biological aggressiveness (G), its local extent (T), and the presence or absence of metastasis (M). Histological, radiological, and clinical features correlate to identify benign (G0), low-grade malignant (G1), or high-grade malignant (G2) lesions. Benign (G0) lesions are then further subcategorized as being latent (stage 1), active (stage 2), or aggressive (stage 3) based on characteristics of the tumor host margin. Stage 1 benign lesions are slow growing or static, and characterized by mature fibrous tissue or cortical bone encapsulation. Stage 2 benign lesions grow steadily and are bordered by a thin capsule surrounded by an area of reactive tissue. Stage 3 benign tumors extend rapidly, usually preceded by a thick pseudocapsule of reactive tissue with a penetrated or absent capsule. The location of the tumor is categorized as intracapsular (T0), intracompartmental (T1), or extracompartmental (T2). Absence of metastases is denoted as M0 and the presence of distant metastasis as M1. These three factors combine to create the Enneking stage (Table 12.1). For each stage, a specific resection margin is recommended (Table 12.2). Adhering to and respecting the Enneking principles for tumors of the appendicular skeleton has been shown to result in a lower rate of recurrence and in improved survival. During recent decades, advancements in imaging and surgical technology along with spinal oncology subspecialization has permitted the safe application of the Enneking principles for tumors of the spinal column. As has been demonstrated in the appendicular skeleton, this standardized approach has been shown to achieve acceptable morbidity, mortality, and health-related quality of life outcomes for primary spine tumors.2–4 In a multicenter ambispective cohort analysis of 147 patients with primary spine tumors, Fisher et al5 demonstrated that the adoption of Enneking principles resulted in a significant reduction in rates of local recurrence and improved life expectancy. Studies assessing the recurrence risk and tumor-free survival following surgical resection of spinal column chordomas6,7 and chondrosarcomas8 have also confirmed the importance of adhering to Enneking fundamentals. Table 12.2 Modified Articulation of Enneking Stages with Surgical Margins
Principles Behind Determining the Right Approach

 Introduction
Introduction
 Enneking Classification
Enneking Classification
| Enneking Stages | Margin for Control |
| 1 | No management unless for decompression or stabilization |
| 2 | Intralesional excision ± local adjuvants |
| 3 | Marginal en-bloc excision |
| 1a | Wide en-bloc excision |
| 1b | Wide en-bloc excision |
| 2a | Wide en-bloc excision + effective adjuvants |
| 2b | Wide en-bloc excision + effective adjuvants |
| 3a | Palliative |
| 3b | Palliative |
Source: From Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009;34:385. Reproduced with permission.)
For spinal column tumors, the Enneking classification does not take into account the presence of a continuous epidural compartment, the neurologic implications, and the need for restoring spinal stability.9 Furthermore, the size of the tumor, which has been found to be correlated with adverse prognosis,6 is not considered. The system was originally based on the natural history of mesenchymal tumors and thus is not applicable to tumors originating from bone marrow, the reticuloendothelial system, or metastatic carcinomas.
 Weinstein-Boriani-Biagini Classification
Weinstein-Boriani-Biagini Classification
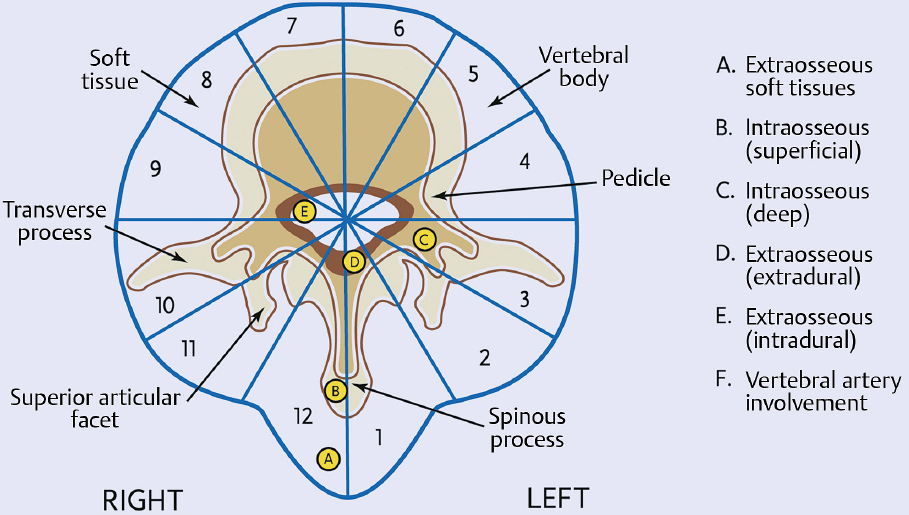
The Weinstein-Boriani-Biagini (WBB) classification incorporates the Enneking principles into the staging and surgical approach of primary tumors of the spine by addressing the unique complexity of lesions in this anatomic setting.10 The pioneering work of these authors significantly assists surgical planning by establishing feasibility criteria and strategies for achieving oncological resection of these tumors while sparing the neurologic elements based on the location of the lesion. In this surgical staging system, vertebral bodies are divided into 12 equal radiating zones in the axial plane (Fig. 12.1) numbered clockwise from the left side of the spinous process (1) to the right side of the spinous process (12). The tumor is further divided into five concentric layers centered about the dural sac: A, extraosseous soft tissues; B, intraosseous superficial; C, intraosseous deep; D, (extraosseous extradural; and E, extraosseous intradural. Finally, the longitudinal extent of the tumor is recorded as the number of vertebral segments involved. Based on the WBB stages, Boriani et al4 proposed indications for surgical procedures based on their experience with 29 patients.
For tumors of the spinal column, moderate interobserver reliability and substantial near-perfect intraobserver reliability for both the Enneking and WBB classification, in terms of staging and guidance for treatment, have been reported.11
Fig. 12.1 Weinstein-Boriani-Biagini (WBB) surgical staging of spine tumors. (From Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine 1996;21:1927–1931. Reproduced with permission.)
 Surgical Margins
Surgical Margins
Standardized terminology, which has been a source of confusion in the literature, is fundamental for the study of primary spinal column tumors. Curettage or piecemeal resection refers to deliberate intralesionnal resection. En-bloc resection, on the other hand, signifies an attempt to resect a tumor in one piece. En-bloc resection on its own has no clear signification without an appropriate histological description of the resection margins (intralesional, marginal, wide, or radical). An intralesional margin denotes that the plane of dissection has transgressed into the lesion. A marginal margin refers to dissection within the reactive zone or pseudocapsule surrounding the tumor, whereas a wide resection margin signifies the dissection has occurred beyond the reactive zone through normal tissue. In spine surgery, radical margins, which refer to extracompartmental resection, are rarely feasible, as the epidural space is considered a continuous compartment extending from occiput to the sacrum. The only theoretical scenario is a stage I/IIA tumor totally confined to the vertebra (without epidural disease) where a complete resection including the spinal cord is performed. Achieving wide resection margins in tumors involving the epidural compartment would imply dissection of dural tissue, which would significantly complicate the approach and increase the complication rate. Consequently when there is epidural tumor, marginal resection at the dura is often performed. The cost–benefit ratio of achieving a wide resection at the dura has not been fully evaluated.
When preservation of the uninvolved neural elements cannot be achieved without compromising the surgical margins, the surgeon, conjointly with the patient, has to decide on a compromise. Creating a neurologic deficit can be justified to achieve a potential cure in a patient with a long life expectancy. The alternative is to compromise the Enneking surgical principles and perform a planned tumor transgression to enable delivery of the tumor without neural element sacrifice. Compromising the surgical margins in a planned, predictable fashion has the potential to cause less tumor spillage and a lower rate of local recurrence than a deliberate intralesionnal resection. It cannot be overstated that these decisions must include the patient’s personal preferences following a thorough discussion of options. Chapter 11 discusses the issues of surgical margins and planned transgression in detail.
 Determining the Diagnosis
Determining the Diagnosis
The approach to primary spinal tumors begins with determining the appropriate histological diagnosis. Because of the rarity of these tumors, a pathologist experienced with diagnosing these conditions should be consulted. The differential diagnosis includes primary mesenchymal tumors, primary nonmesenchymal tumor (hematological or reticuloendothelial system malignancies), metastatic disease, pyogenic and nonpyogenic spinal infections, inflammatory processes, as well as the more common traumatic and degenerative pathologies. The multitude of primary tumor pathologies and their varying intrinsic biological behaviors make determining an accurate diagnosis of the upmost importance. Ewing’s sarcomas, for example, can respond strikingly to neoadjuvant therapies and can thus be considered for medical therapy only.
How tissue is obtained to facilitate a histological diagnosis can have a significant influence on patient outcomes and the surgical strategy subsequently selected. A systematic review addressed this issue.3 A computed tomography (CT)-guided trocar biopsy appears to be the best oncological way of safely determining a diagnosis, as incisional or open biopsies have been associated with higher recurrence rates and lower disease-free survival. When a primary spinal tumor is suspected, an oncological spine surgeon should be involved early, as the biopsy tract needs to be marked to ensure its inclusion during the definitive resection.
 Multidisciplinary Teams
Multidisciplinary Teams
Assembling a multidisciplinary team for preoperative decision making and planning is fundamental to achieving optimal primary spine tumor management. Each case should be discussed in rounds attended by an oncological spine surgeon, radiation oncologist, medical oncologist, pathologist, and radiologist. A group review of the local and systemic workup, histological diagnosis, and staging will enable consensus conclusions to be reached on key issues including the role and feasibility of surgery, and the suitability of neoadjuvant and adjuvant therapies. If a neoadjuvant treatment is deemed necessary, restaging is recommended after completion, before contemplating surgical treatment.
When resection is indicated, various surgical subspecialties can play significant supporting roles to the spine surgeon. When considerable vascular manipulation or reconstruction is necessary, a vascular surgeon is indispensable. Plastic surgeons can assist with the closure of precarious wounds or perform musculocutaneous flap coverage when necessary. When abdominal or pelvic contents and the tumor are closely approximated, a general surgeon can assist in the dissection and create derivation colostomies when required. Complex dissections of the neck can be facilitated by head and neck surgeons, whereas dissection within the chest cavity can be assisted by thoracic surgeons.
 Surgical Planning
Surgical Planning
Because each primary spine tumor has unique characteristics and localizations, surgeons must be flexible about determining the right approach. Each case should be approached individually. The choice of surgical margins according to the Enneking principles is the first step in planning surgical intervention (Table 12.2). With advancements in surgical expertise, imaging and technology are important adjuncts in determining the surgical approach to any spinal tumor. We believe there should not be any preset approaches based on localization.
En-bloc resection for tumors of the spinal column was first described in the 1960s. Lièvre et al12 described a two-stage en-bloc resection of an L4 giant cell tumor. The first stage consisted of resection of the posterior spinal elements, followed by an anterior vertebral body resection 2 weeks later. In the following years, Stener described a similar approach for a giant cell tumor of T11-L113and later reported on his extensive experience (1968–1981) with complete removal of vertebrae for extirpation of tumors in 23 consecutive patients with a minimum of 7-year follow-up.14 The basic principles used in his case series are still used today.
Stener pioneered the fundamental principle of considering the spinal column to be like a ring through which the neural elements pass. To achieve a resection while adhering to Enneking’s principle and preserving neurologic function, a tumor-free window has to be created in that “ring” through which the spinal cord can be delivered during tumor removal. The window can be created by piecemeal resection because it involves nontumoral tissue. A second fundamental principle is to have access to the nerve root at the dural margin via a clear plane of dissection between the tumor and the dura when amputation of a root is necessary. This is often where only a marginal resection can be accomplished, but it is essential for the delivery of the tumor. Another area at risk of oncological contamination is the pedicle.
The localization of the tumor within the “ring” will dictate the feasibility, approach, and method by which the tumor is delivered during en-bloc resection. Boriani et al10 described three common scenarios for thoracolumbar lesions. First, an en-bloc resection can be performed with an appropriate margin if the tumor is localized in zones 4 to 8 or 5 to 9 of the WBB staging system. In other words, the posterior elements and at least one pedicle have to be free of tumor to be able to achieve oncological resection while safely delivering the spinal cord. This can be achieved via a single posterior or via a staged posteroanterior approach.15 Second, a sagittal resection can be accomplished when the tumor is confined to zones 3 to 5 or 8 to 10. Again, this can be completed through a single posterior or an anteroposterior approach. Third, when the tumor is confined to the posterior elements only (zones 10 to 3), en-bloc resection can be accomplished using a posterior approach only (Table 12.3).
Tumors located in the cervical spine pose a significant challenge because of the many unique anatomic features of this region. The close proximity of the vertebral artery, the peculiar bony architecture, and the functional importance of the cervical roots all make en-bloc resection of a cervical spine tumor demanding. Although the feasibility of en-bloc resection of primary tumors of the cervical spine has been demonstrated, because of these anatomic issues significant morbidity and complications are frequent.16–19 Among others, prolonged intubation, acute respiratory distress syndrome, dehiscence of the posterior pharyngeal wall, prolonged dysphagia, and hardware failure requiring revision have all been reported.17,19 In the decision-making process these adverse events have to be plotted against the dismal natural history of these tumors and have to be discussed candidly with the patient.
Table 12.3 Articulation of Weinstein-Boriani-Biagini (WBB) Stages with Surgical Procedures
| Radiating Zone | Procedure |
| 4–8 or 5–9 | Vertebrectomy (double approach) |
| 2–5 or 7–11 | Sagittal resection (double approach) |
| 10–3 | Posterior arch resection (posterior approach) |
Source: From Chan P, Boriani S, Fourney DR, et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009;34:385. Reproduced with permission.)
The consequences of unilateral vertebral artery resection are dependent on the variable anatomy of the vertebrobasilar system.20 An endovascular balloon-occlusion test, cerebral blood-flow studies, and intraoperative temporary occlusion with neuromonitoring are strategies for assessing whether sacrifice can be performed without significant ischemic compromise.
Although the resection of cervical nerve roots frequently carries significant morbidity, in selected cases it can be tolerated. Bailey et al19 reported no clinically significant consequences following unilateral C2 to C4 nerve root sacrifice despite potential hemidiaphragm paralysis.
Tumors located in the sacrum are associated with a different set of surgical considerations. From a neurologic perspective, sacral roots often need to be sacrificed to achieve a true en-bloc oncological resection, putting bowel, bladder, and sexual function at risk. Todd et al21 showed that preservation of bowel and bladder continence after major sacral resection occurs in the majority of patients following unilateral sacral root resection or if at least one S3 root is preserved in the case of bilateral sacral resection. Other considerations include the close proximity of major vessels anteriorly and the risk of significant blood loss. The close proximity of the anus and the risk of bowel perforation also increase the risk of surgical-site infection. Derivation colostomies, especially when loss of bowel function is anticipated, have been used to mitigate this occurrence. Wide resection also results in large soft tissue defects. Rotational gluteal or transpelvic vertical rectus abdominis myocutaneous (VRAM) flaps can be used to obliterate this dead space and improve wound healing.22 Lastly, high sacral resections can interfere with spinopelvic stability, necessitating complex reconstructive techniques (see Chapter 13).
At our institution, preoperative angiography and embolization is routinely attempted. This enhances knowledge of the vascular anatomy and helps with operative planning and the management of intraoperative blood loss. Even though tumoral bleeding should not be a concern if tumor violation is avoided, embolization of involved radicular arteries in the thoracolumbar spine and the vertebral artery in the cervical spine, as well as major tumor feeders, has been effective in reducing blood loss–related complications.
 Illustrative Cases
Illustrative Cases
Case 1
History and Examination
A 50-year-old Asian man presented with an 18-month history of progressive right-sided clumsiness, weakness, and numbness, starting initially in his right arm then progressing to involve his right leg. Examination demonstrated bilateral upper extremity altered sensation as well as hyperreflexia and gait disturbance consistent with myelopathy but without objective motor deficit.19
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree